Impaired calcium signaling in astrocytes modulates autism spectrum disorder-like behaviors in mice
- PMID: 34059669
- PMCID: PMC8166865
- DOI: 10.1038/s41467-021-23843-0
Impaired calcium signaling in astrocytes modulates autism spectrum disorder-like behaviors in mice
Abstract
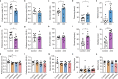
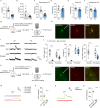
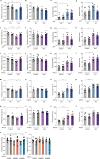
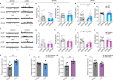
Autism spectrum disorder (ASD) is a common neurodevelopmental disorder. The mechanisms underlying ASD are unclear. Astrocyte alterations are noted in ASD patients and animal models. However, whether astrocyte dysfunction is causal or consequential to ASD-like phenotypes in mice is unresolved. Type 2 inositol 1,4,5-trisphosphate 6 receptors (IP3R2)-mediated Ca2+ release from intracellular Ca2+ stores results in the activation of astrocytes. Mutations of the IP3R2 gene are associated with ASD. Here, we show that both IP3R2-null mutant mice and astrocyte-specific IP3R2 conditional knockout mice display ASD-like behaviors, such as atypical social interaction and repetitive behavior. Furthermore, we show that astrocyte-derived ATP modulates ASD-like behavior through the P2X2 receptors in the prefrontal cortex and possibly through GABAergic synaptic transmission. These findings identify astrocyte-derived ATP as a potential molecular player in the pathophysiology of ASD.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The IP3R2 Knockout Mice in Behavior: A Blessing or a Curse?J Neurochem. 2025 Apr;169(4):e70062. doi: 10.1111/jnc.70062. J Neurochem. 2025. PMID: 40172184 Review.
-
Disruption of IP₃R2-mediated Ca²⁺ signaling pathway in astrocytes ameliorates neuronal death and brain damage while reducing behavioral deficits after focal ischemic stroke.Cell Calcium. 2015 Dec;58(6):565-76. doi: 10.1016/j.ceca.2015.09.004. Epub 2015 Sep 25. Cell Calcium. 2015. PMID: 26433454 Free PMC article.
-
Ca(2+) signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo.Nat Neurosci. 2015 May;18(5):708-17. doi: 10.1038/nn.4001. Epub 2015 Apr 20. Nat Neurosci. 2015. PMID: 25894291 Free PMC article.
-
A Novel Human CAMK2A Mutation Disrupts Dendritic Morphology and Synaptic Transmission, and Causes ASD-Related Behaviors.J Neurosci. 2017 Feb 22;37(8):2216-2233. doi: 10.1523/JNEUROSCI.2068-16.2017. Epub 2017 Jan 27. J Neurosci. 2017. PMID: 28130356 Free PMC article.
-
The Interplay of Astrocytes and Neurons in Autism Spectrum Disorder.Adv Neurobiol. 2024;39:269-284. doi: 10.1007/978-3-031-64839-7_11. Adv Neurobiol. 2024. PMID: 39190079 Review.
Cited by
-
Revisiting astrocytic calcium signaling in the brain.Fundam Res. 2024 Feb 8;4(6):1365-1374. doi: 10.1016/j.fmre.2023.11.021. eCollection 2024 Nov. Fundam Res. 2024. PMID: 39734522 Free PMC article. Review.
-
Neurodevelopmental disorders: 2022 update.Free Neuropathol. 2022 Mar 21;3:8. doi: 10.17879/freeneuropathology-2022-3801. eCollection 2022 Jan. Free Neuropathol. 2022. PMID: 37284163 Free PMC article.
-
Astrocyte metabolism and signaling pathways in the CNS.Front Neurosci. 2023 Sep 4;17:1217451. doi: 10.3389/fnins.2023.1217451. eCollection 2023. Front Neurosci. 2023. PMID: 37732313 Free PMC article. Review.
-
High urea promotes mitochondrial fission and functional impairments in astrocytes inducing anxiety-like behavior in chronic kidney disease mice.Metab Brain Dis. 2025 Apr 17;40(5):186. doi: 10.1007/s11011-025-01612-y. Metab Brain Dis. 2025. PMID: 40244426
-
IP3R2-Mediated Astrocytic Ca2+ Transients Are Critical to Sustain Modulatory Effects of Locomotion on Neurons in Mouse Somatosensory Cortex.Cells. 2025 Jul 18;14(14):1103. doi: 10.3390/cells14141103. Cells. 2025. PMID: 40710356 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

