DHT causes liver steatosis via transcriptional regulation of SCAP in normal weight female mice
- PMID: 34060475
- PMCID: PMC8240729
- DOI: 10.1530/JOE-21-0040
DHT causes liver steatosis via transcriptional regulation of SCAP in normal weight female mice
Abstract
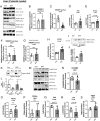
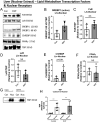
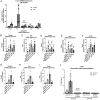
Hyperandrogenemia (HA) is a hallmark of polycystic ovary syndrome (PCOS) and is an integral element of non-alcoholic fatty liver disease (NALFD) in females. Administering low-dose dihydrotestosterone (DHT) induced a normal weight PCOS-like female mouse model displaying NAFLD. The molecular mechanism of HA-induced NAFLD has not been fully determined. We hypothesized that DHT would regulate hepatic lipid metabolism via increased SREBP1 expression leading to NAFLD. We extracted liver from control and low-dose DHT female mice; and performed histological and biochemical lipid profiles, Western blot, immunoprecipitation, chromatin immunoprecipitation, and real-time quantitative PCR analyses. DHT lowered the 65 kD form of cytosolic SREBP1 in the liver compared to controls. However, DHT did not alter the levels of SREBP2 in the liver. DHT mice displayed increased SCAP protein expression and SCAP-SREBP1 binding compared to controls. DHT mice exhibited increased AR binding to intron-8 of SCAP leading to increased SCAP mRNA compared to controls. FAS mRNA and protein expression was increased in the liver of DHT mice compared to controls. p-ACC levels were unaltered in the liver. Other lipid metabolism pathways were examined in the liver, but no changes were observed. Our findings support evidence that DHT increased de novo lipogenic proteins resulting in increased hepatic lipid content via regulation of SREBP1 in the liver. We show that in the presence of DHT, the SCAP-SREBP1 interaction was elevated leading to increased nuclear SREBP1 resulting in increased de novo lipogenesis. We propose that the mechanism of action may be increased AR binding to an ARE in SCAP intron-8.
Keywords: SREBP; androgen signaling; hyperandrogenemia or androgen excess; lipogenesis.
Figures


Similar articles
-
CD36 promotes de novo lipogenesis in hepatocytes through INSIG2-dependent SREBP1 processing.Mol Metab. 2022 Mar;57:101428. doi: 10.1016/j.molmet.2021.101428. Epub 2021 Dec 30. Mol Metab. 2022. PMID: 34974159 Free PMC article.
-
Regulated expression of the prostacyclin receptor (IP) gene by androgens within the vasculature: Combined role for androgens and serum cholesterol.Biochim Biophys Acta. 2016 Oct;1859(10):1333-51. doi: 10.1016/j.bbagrm.2016.06.011. Epub 2016 Jun 27. Biochim Biophys Acta. 2016. PMID: 27365208
-
The role of KLF2 in regulating hepatic lipogenesis and blood cholesterol homeostasis via the SCAP/SREBP pathway.J Lipid Res. 2024 Jan;65(1):100472. doi: 10.1016/j.jlr.2023.100472. Epub 2023 Nov 9. J Lipid Res. 2024. PMID: 37949368 Free PMC article.
-
The Role of SCAP/SREBP as Central Regulators of Lipid Metabolism in Hepatic Steatosis.Int J Mol Sci. 2024 Jan 16;25(2):1109. doi: 10.3390/ijms25021109. Int J Mol Sci. 2024. PMID: 38256181 Free PMC article. Review.
-
The cellular function of SCAP in metabolic signaling.Exp Mol Med. 2020 May;52(5):724-729. doi: 10.1038/s12276-020-0430-0. Epub 2020 May 8. Exp Mol Med. 2020. PMID: 32385422 Free PMC article. Review.
Cited by
-
Liver Androgen Receptor Knockout Improved High-fat Diet Induced Glucose Dysregulation in Female Mice But Not Male Mice.J Endocr Soc. 2024 Feb 9;8(4):bvae021. doi: 10.1210/jendso/bvae021. eCollection 2024 Feb 19. J Endocr Soc. 2024. PMID: 38425436 Free PMC article.
-
Non-alcoholic fatty liver disease in women - Current knowledge and emerging concepts.JHEP Rep. 2023 Jun 29;5(10):100835. doi: 10.1016/j.jhepr.2023.100835. eCollection 2023 Oct. JHEP Rep. 2023. PMID: 37771547 Free PMC article. Review.
-
Regulation and targeting of SREBP-1 in hepatocellular carcinoma.Cancer Metastasis Rev. 2024 Jun;43(2):673-708. doi: 10.1007/s10555-023-10156-5. Epub 2023 Dec 1. Cancer Metastasis Rev. 2024. PMID: 38036934 Free PMC article. Review.
-
Biochemical, sex hormonal, and anthropometric predictors of non-alcoholic fatty liver disease in polycystic ovary syndrome.BMC Womens Health. 2025 Mar 14;25(1):118. doi: 10.1186/s12905-025-03648-9. BMC Womens Health. 2025. PMID: 40087649 Free PMC article.
-
Mechanism of Metabolic Dysfunction-associated Steatotic Liver Disease: Important role of lipid metabolism.J Clin Transl Hepatol. 2024 Sep 28;12(9):815-826. doi: 10.14218/JCTH.2024.00019. Epub 2024 Sep 3. J Clin Transl Hepatol. 2024. PMID: 39280069 Free PMC article. Review.
References
-
- Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, Mcguinness OP. & NIH Mouse Metabolic Phenotyping Center Consortium 2010. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Disease Models and Mechanisms 3 525–534. (10.1242/dmm.006239) - DOI - PMC - PubMed
-
- Baranova A, Tran TP, Afendy A, Wang L, Shamsaddini A, Mehta R, Chandhoke V, Birerdinc A, Younossi ZM. 2013. Molecular signature of adipose tissue in patients with both non-alcoholic fatty liver disease (NAFLD) and polycystic ovarian syndrome (PCOS). Journal of Translational Medicine 11 133. (10.1186/1479-5876-11-133) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

