Tumor innervation: peripheral nerves take control of the tumor microenvironment
- PMID: 34060481
- PMCID: PMC8159682
- DOI: 10.1172/JCI147276
Tumor innervation: peripheral nerves take control of the tumor microenvironment
Abstract
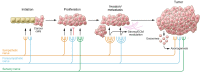
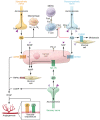
In recent decades, cancer research has expanded exponentially beyond the study of abnormally dividing cells to include complex and extensive heterotypic interactions between cancer and noncancer cells that constitute the tumor microenvironment (TME). Modulation of stromal, immune, and endothelial cells by cancer cells promotes proliferation, survival, and metabolic changes that support tumor growth and metastasis. Recent evidence demonstrates that tumors can recruit peripheral nerves to the TME, leading to enhanced tumor growth in a range of cancer models through distinct mechanisms. This process, termed tumor innervation, is associated with an aggressive tumor phenotype and correlates with poor prognosis in clinical studies. Therefore, the peripheral nervous system may play an underrecognized role in cancer development, harboring targetable pathways that warrant investigation. To date, nerves have been implicated in driving proliferation, invasion, metastasis, and immune evasion through locally delivered neurotransmitters. However, emerging evidence suggests that cell-cell communication via exosomes induces tumor innervation, and thus exosomes may also mediate neural regulation of the TME. In this Review, seminal studies establishing tumor innervation are discussed, and known and putative signaling mechanisms between peripheral nerves and components of the TME are explored as a means to identify potential opportunities for therapeutic intervention.
Conflict of interest statement
Figures