Ironing out mechanisms of iron homeostasis and disorders of iron deficiency
- PMID: 34060484
- PMCID: PMC8159681
- DOI: 10.1172/JCI148671
Ironing out mechanisms of iron homeostasis and disorders of iron deficiency
Abstract
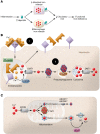
Iron plays an important role in mammalian physiological processes. It is a critical component for the function of many proteins, including enzymes that require heme and iron-sulfur clusters. However, excess iron is also detrimental because of its ability to catalyze the formation of reactive oxygen species. As a result, cellular and systemic iron levels are tightly regulated to prevent oxidative damage. Iron deficiency can lead to a number of pathological conditions, the most prominent being anemia. Iron deficiency should be corrected to improve adult patients' symptoms and to facilitate normal growth during fetal development and childhood. However, inappropriate use of intravenous iron in chronic conditions, such as cancer and heart failure, in the absence of clear iron deficiency can lead to unwanted side effects. Thus, this form of therapy should be reserved for certain patients who cannot tolerate oral iron and need rapid iron replenishment. Here, we will review cellular and systemic iron homeostasis and will discuss complications of iron deficiency.
Conflict of interest statement
Figures


Comment in
-
The iron revolution: Keeping abreast of the developments in iron therapy.Am J Hematol. 2022 Mar 1;97(3):250-252. doi: 10.1002/ajh.26427. Epub 2021 Dec 8. Am J Hematol. 2022. PMID: 34856013 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

