Nanomechanical Phenotypes in Cardiac Myosin-Binding Protein C Mutants That Cause Hypertrophic Cardiomyopathy
- PMID: 34060810
- PMCID: PMC8514129
- DOI: 10.1021/acsnano.1c02242
Nanomechanical Phenotypes in Cardiac Myosin-Binding Protein C Mutants That Cause Hypertrophic Cardiomyopathy
Abstract
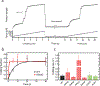
Hypertrophic cardiomyopathy (HCM) is a disease of the myocardium caused by mutations in sarcomeric proteins with mechanical roles, such as the molecular motor myosin. Around half of the HCM-causing genetic variants target contraction modulator cardiac myosin-binding protein C (cMyBP-C), although the underlying pathogenic mechanisms remain unclear since many of these mutations cause no alterations in protein structure and stability. As an alternative pathomechanism, here we have examined whether pathogenic mutations perturb the nanomechanics of cMyBP-C, which would compromise its modulatory mechanical tethers across sliding actomyosin filaments. Using single-molecule atomic force spectroscopy, we have quantified mechanical folding and unfolding transitions in cMyBP-C domains targeted by HCM mutations that do not induce RNA splicing alterations or protein thermodynamic destabilization. Our results show that domains containing mutation R495W are mechanically weaker than wild-type at forces below 40 pN and that R502Q mutant domains fold faster than wild-type. None of these alterations are found in control, nonpathogenic variants, suggesting that nanomechanical phenotypes induced by pathogenic cMyBP-C mutations contribute to HCM development. We propose that mutation-induced nanomechanical alterations may be common in mechanical proteins involved in human pathologies.
Keywords: AFM; cMyBP-C; contraction; hypertrophic cardiomyopathy; protein mechanics; single-molecule.
Conflict of interest statement
The authors declare the following competing financial interest(s): L.M. is a share-holder of Health in Code. J.A.S. is a Co-founder of, consults for, and owns stock in Cytokinetics, Inc.
A version of the manuscript has been submitted to a pre-print server.
Figures
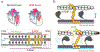
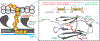



Similar articles
-
The mechanics of the heart: zooming in on hypertrophic cardiomyopathy and cMyBP-C.FEBS Lett. 2022 Mar;596(6):703-746. doi: 10.1002/1873-3468.14301. Epub 2022 Feb 28. FEBS Lett. 2022. PMID: 35224729 Review.
-
E258K HCM-causing mutation in cardiac MyBP-C reduces contractile force and accelerates twitch kinetics by disrupting the cMyBP-C and myosin S2 interaction.J Gen Physiol. 2013 Sep;142(3):241-55. doi: 10.1085/jgp.201311018. J Gen Physiol. 2013. PMID: 23980194 Free PMC article.
-
The W792R HCM missense mutation in the C6 domain of cardiac myosin binding protein-C increases contractility in neonatal mouse myocardium.J Mol Cell Cardiol. 2024 Oct;195:14-23. doi: 10.1016/j.yjmcc.2024.07.007. Epub 2024 Jul 25. J Mol Cell Cardiol. 2024. PMID: 39059462
-
A hypertrophic cardiomyopathy-associated MYBPC3 mutation common in populations of South Asian descent causes contractile dysfunction.J Biol Chem. 2015 Feb 27;290(9):5855-67. doi: 10.1074/jbc.M114.607911. Epub 2015 Jan 12. J Biol Chem. 2015. PMID: 25583989 Free PMC article.
-
Cardiac myosin-binding protein C in hypertrophic cardiomyopathy: mechanisms and therapeutic opportunities.J Mol Cell Cardiol. 2011 Apr;50(4):613-20. doi: 10.1016/j.yjmcc.2011.01.014. Epub 2011 Feb 1. J Mol Cell Cardiol. 2011. PMID: 21291890 Review.
Cited by
-
An Update on MYBPC3 Gene Mutation in Hypertrophic Cardiomyopathy.Int J Mol Sci. 2023 Jun 22;24(13):10510. doi: 10.3390/ijms241310510. Int J Mol Sci. 2023. PMID: 37445689 Free PMC article. Review.
-
Assessment of the Contribution of a Thermodynamic and Mechanical Destabilization of Myosin-Binding Protein C Domain C2 to the Pathomechanism of Hypertrophic Cardiomyopathy-Causing Double Mutation MYBPC3Δ25bp/D389V.Int J Mol Sci. 2021 Nov 4;22(21):11949. doi: 10.3390/ijms222111949. Int J Mol Sci. 2021. PMID: 34769381 Free PMC article.
-
Molecular Mechanism of Interaction between DNA Aptamer and Receptor-Binding Domain of Severe Acute Respiratory Syndrome Coronavirus 2 Variants Revealed by Steered Molecular Dynamics Simulations.Molecules. 2024 May 9;29(10):2215. doi: 10.3390/molecules29102215. Molecules. 2024. PMID: 38792076 Free PMC article.
-
High-throughput virtual search of small molecules for controlling the mechanical stability of human CD4.J Biol Chem. 2024 Apr;300(4):107133. doi: 10.1016/j.jbc.2024.107133. Epub 2024 Mar 2. J Biol Chem. 2024. PMID: 38432632 Free PMC article.
-
Single-Molecule Force Spectroscopy Reveals Stability of mitoNEET and its [2Fe2Se] Cluster in Weakly Acidic and Basic Solutions.ChemistryOpen. 2022 May;11(5):e202200056. doi: 10.1002/open.202200056. ChemistryOpen. 2022. PMID: 35608094 Free PMC article.
References
-
- Gautel M; Djinovic-Carugo K The Sarcomeric Cytoskeleton: From Molecules to Motion. J. Exp. Biol 2016, 219, 135–145. - PubMed
-
- Braunwald E Hypertrophic Cardiomyopathy. In Hypertrophic Cardiomyopathy: Foreword by Bernard Gersh and Historical Context by Eugene Braunwald; Springer-Verlag: London, 2015; pp 1–8.
-
- Semsarian C; Ingles J; Maron MS; Maron BJ New Perspectives on the Prevalence of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol 2015, 65, 1249–1254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

