An Organometallic Strategy for Cysteine Borylation
- PMID: 34060827
- PMCID: PMC8437308
- DOI: 10.1021/jacs.1c02206
An Organometallic Strategy for Cysteine Borylation
Abstract
Synthetic bioconjugation at cysteine (Cys) residues in peptides and proteins has emerged as a powerful tool in chemistry. Soft nucleophilicity of the sulfur in Cys renders an exquisite chemoselectivity with which various functional groups can be placed onto this residue under benign conditions. While a variety of reactions have been successful at producing Cys-based bioconjugates, the majority of these feature sulfur-carbon bonds. We report Cys-borylation, wherein a benchtop stable Pt(II)-based organometallic reagent can be used to transfer a boron-rich cluster onto a sulfur moiety in unprotected peptides forging a boron-sulfur bond. Cys-borylation proceeds at room temperature and tolerates a variety of functional groups present in complex polypeptides. Further, the bioconjugation strategy can be applied to a model protein modification of Cys-containing DARPin (designed ankyrin repeat protein). The resultant bioconjugates show no additional toxicity compared to their Cys alkyl-based congeners. Finally, we demonstrate how the developed Cys-borylation can enhance the proteolytic stability of the resultant peptide bioconjugates while maintaining the binding affinity to a protein target.
Conflict of interest statement
Notes
The authors declare no competing financial interests.
Figures



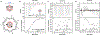

References
-
- Hermanson GT Bioconjugate Techniques, 3rd ed.; Academic Press: San Diego, CA, 2013.
-
- Recent representative reviews on general methods: Stephanopoulos N & Francis MB Choosing an Effective Protein Bioconjugation Strategy. Nature Chem. Bio 2011, 7, 876–884. - PubMed
- McKay CS & Finn MG Click Chemistry in Complex Mixtures: Biorthogonal Bioconjugation. Chem. Biol 2014, 21, 1075–1101. - PMC - PubMed
- Boutureira O & Bernardes GJL Advances in Chemical Protein Modification. Chem. Rev 2015, 115, 2174–2195. - PubMed
- Albaba B & Metzler-Nolte N Organometallic-Peptide Bioconjugates: Synthetic Strategies and Medicinal Applications. Chem. Rev 2016, 116, 11797–11839. - PubMed
- Hu Q-Y; Berti F; Adamo R Towards the Next Generation of Biomedicines by Site-Selective Conjugation. Chem. Soc. Rev 2016, 45, 1691–1719. - PubMed
- Vinogradova EV Organometallic Chemical Biology: An Organometallic Approach to Bioconjugation. Pure & Appl. Chem 2017, 89, 1619–1640.
- Jbara M; Maity SK; Brik A Palladium in the Chemical Synthesis and Modification of Proteins. Angew. Chem., Int. Ed 2017, 56, 10644–10655. - PubMed
- Ohata J; Martin SC; Ball ZT Metal-Mediated Functionalization of Natural Peptides and Proteins: Panning for Bioconjugation Gold. Angew. Chem., Int. Ed 2019, 58, 6176–6199. - PubMed
- Conibear AC; Watson EE; Payne RJ; Becker CFW Native Chemical Ligation in Protein Synthesis and Semi-Synthesis. Chem. Soc. Rev 2019, 47, 9046–9068. - PubMed
- Isenegger PG & Davis BG Concepts of catalysis in site-selective protein modifications. J. Am. Chem. Soc 2020, 141, 8005–8013. - PMC - PubMed
- Latocheski E; Dal Forno GM; Ferreira TM; Oliveira BL; Bernardes GJL; Domingos JB Mechanistic insights into transition metal-mediated bioorthogonal uncaging reactions Chem. Soc. Rev 2020, 49, 7710–7729. - PubMed
- Szijj PA; Kostadinova KA; Spears RJ; Chudasama V Tyrosine Bioconjugation - An Emergent Alternative. Org. Biomol. Chem 2020, 18, 9018–9028. - PMC - PubMed
- Patra M & Gasser G Organometallic Compounds: An Opportunity for Chemical Biology? ChemBioChem 2021, 13, 1232–1252. - PubMed
-
- Lavis LD Teaching Old Dyes New Tricks: Biological Probes Built from Fluoresceins and Rhodamines. Annu. Rev. Biochem 2017, 86, 825–43. - PubMed
-
- Li Y Commonly Used Tag Combinations for Tandem Affinity Purification. Biotechnol. Appl. Biochem 2010, 55, 73–83. - PubMed
-
- Liu H; Bolleddula J; Nichols A; Tang L; Zhao Z; Prakash C Metabolism of Bioconjugate Therapeutics: Why, When and How? Drug Metab. Rev 2020, 52, 66–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

