Effects of Combined and Alone Transcranial Motor Cortex Stimulation and Mirror Therapy in Phantom Limb Pain: A Randomized Factorial Trial
- PMID: 34060934
- PMCID: PMC10042175
- DOI: 10.1177/15459683211017509
Effects of Combined and Alone Transcranial Motor Cortex Stimulation and Mirror Therapy in Phantom Limb Pain: A Randomized Factorial Trial
Abstract
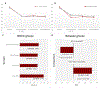
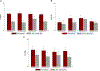
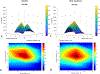
Phantom limb pain (PLP) is a frequent complication in amputees, which is often refractory to treatments. We aim to assess in a factorial trial the effects of transcranial direct current stimulation (tDCS) and mirror therapy (MT) in patients with traumatic lower limb amputation; and whether the motor cortex plasticity changes drive these results. In this large randomized, blinded, 2-site, sham-controlled, 2 × 2 factorial trial, 112 participants with traumatic lower limb amputation were randomized into treatment groups. The interventions were active or covered MT for 4 weeks (20 sessions, 15 minutes each) combined with 2 weeks of either active or sham tDCS (10 sessions, 20 minutes each) applied to the contralateral primary motor cortex. The primary outcome was PLP changes on the visual analogue scale at the end of interventions (4 weeks). Motor cortex excitability and cortical mapping were assessed by transcranial magnetic stimulation (TMS). We found no interaction between tDCS and MT groups (F = 1.90, P = .13). In the adjusted models, there was a main effect of active tDCS compared to sham tDCS (beta coefficient = -0.99, P = .04) on phantom pain. The overall effect size was 1.19 (95% confidence interval: 0.90, 1.47). No changes in depression and anxiety were found. TDCS intervention was associated with increased intracortical inhibition (coefficient = 0.96, P = .02) and facilitation (coefficient = 2.03, P = .03) as well as a posterolateral shift of the center of gravity in the affected hemisphere. MT induced no motor cortex plasticity changes assessed by TMS. These findings indicate that transcranial motor cortex stimulation might be an affordable and beneficial PLP treatment modality.
Keywords: mirror therapy; motor cortex; phantom limb pain; transcranial direct current stimulation; transcranial magnetic stimulation.
Conflict of interest statement
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

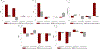
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

