Effect of Long-term Continuous Cardiac Monitoring vs Usual Care on Detection of Atrial Fibrillation in Patients With Stroke Attributed to Large- or Small-Vessel Disease: The STROKE-AF Randomized Clinical Trial
- PMID: 34061145
- PMCID: PMC8170544
- DOI: 10.1001/jama.2021.6470
Effect of Long-term Continuous Cardiac Monitoring vs Usual Care on Detection of Atrial Fibrillation in Patients With Stroke Attributed to Large- or Small-Vessel Disease: The STROKE-AF Randomized Clinical Trial
Abstract
Importance: Patients with ischemic stroke attributed to large- or small-vessel disease are not considered at high risk for atrial fibrillation (AF), and the AF incidence rate in this population is unknown.
Objectives: To determine whether long-term cardiac monitoring is more effective than usual care for AF detection in patients with stroke attributed to large- or small-vessel disease through 12 months of follow-up.
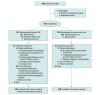
Design, setting, and participants: The STROKE-AF trial was a randomized (1:1), multicenter (33 sites in the US) clinical trial that enrolled 496 patients between April 2016 and July 2019, with primary end point follow-up through August 2020. Eligible patients were aged 60 years or older or aged 50 to 59 years with at least 1 additional stroke risk factor and had an index stroke attributed to large- or small-vessel disease within 10 days prior to insertable cardiac monitor (ICM) insertion.
Interventions: Patients randomized to the intervention group (n = 242) received ICM insertion within 10 days of the index stroke; patients in the control group (n = 250) received site-specific usual care consisting of external cardiac monitoring, such as 12-lead electrocardiograms, Holter monitoring, telemetry, or event recorders.
Main outcomes and measures: Incident AF lasting more than 30 seconds through 12 months.
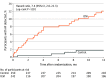
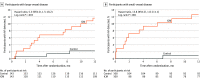
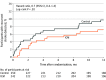
Results: Among 492 patients who were randomized (mean [SD] age, 67.1 [9.4] years; 185 [37.6%] women), 417 (84.8%) completed 12 months of follow-up. The median (interquartile range) CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke or transient ischemic attack, vascular disease, age 65 to 74 years, sex category) score was 5 (4-6). AF detection at 12 months was significantly higher in the ICM group vs the control group (27 patients [12.1%] vs 4 patients [1.8%]; hazard ratio, 7.4 [95% CI, 2.6-21.3]; P < .001). Among the 221 patients in the ICM group who received an ICM, 4 (1.8%) had ICM procedure-related adverse events (1 site infection, 2 incision site hemorrhages, and 1 implant site pain).
Conclusions and relevance: Among patients with stroke attributed to large- or small-vessel disease, monitoring with an ICM compared with usual care detected significantly more AF over 12 months. However, further research is needed to understand whether identifying AF in these patients is of clinical importance.
Trial registration: ClinicalTrials.gov Identifier: NCT02700945.
Conflict of interest statement
Figures
Comment in
-
Detection of Subclinical Atrial Fibrillation After Stroke: Is There Enough Evidence to Treat?JAMA. 2021 Jun 1;325(21):2157-2159. doi: 10.1001/jama.2021.7429. JAMA. 2021. PMID: 34061158 No abstract available.
References
-
- Virani SS, Alonso A, Benjamin EJ, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139-e596. doi:10.1161/CIR.0000000000000757 - DOI - PubMed
-
- Kernan WN, Ovbiagele B, Black HR, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236. doi:10.1161/STR.0000000000000024 - DOI - PubMed
-
- January CT, Wann LS, Calkins H, et al. . 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104-132. doi:10.1016/j.jacc.2019.01.011 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

