Association of Simulated COVID-19 Vaccination and Nonpharmaceutical Interventions With Infections, Hospitalizations, and Mortality
- PMID: 34061203
- PMCID: PMC8170542
- DOI: 10.1001/jamanetworkopen.2021.10782
Association of Simulated COVID-19 Vaccination and Nonpharmaceutical Interventions With Infections, Hospitalizations, and Mortality
Abstract
Importance: Vaccination against SARS-CoV-2 has the potential to significantly reduce transmission and COVID-19 morbidity and mortality. The relative importance of vaccination strategies and nonpharmaceutical interventions (NPIs) is not well understood.
Objective: To assess the association of simulated COVID-19 vaccine efficacy and coverage scenarios with and without NPIs with infections, hospitalizations, and deaths.
Design, setting, and participants: An established agent-based decision analytical model was used to simulate COVID-19 transmission and progression from March 24, 2020, to September 23, 2021. The model simulated COVID-19 spread in North Carolina, a US state of 10.5 million people. A network of 1 017 720 agents was constructed from US Census data to represent the statewide population.
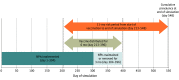
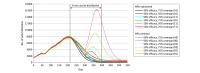
Exposures: Scenarios of vaccine efficacy (50% and 90%), vaccine coverage (25%, 50%, and 75% at the end of a 6-month distribution period), and NPIs (reduced mobility, school closings, and use of face masks) maintained and removed during vaccine distribution.
Main outcomes and measures: Risks of infection from the start of vaccine distribution and risk differences comparing scenarios. Outcome means and SDs were calculated across replications.
Results: In the worst-case vaccination scenario (50% efficacy, 25% coverage), a mean (SD) of 2 231 134 (117 867) new infections occurred after vaccination began with NPIs removed, and a mean (SD) of 799 949 (60 279) new infections occurred with NPIs maintained during 11 months. In contrast, in the best-case scenario (90% efficacy, 75% coverage), a mean (SD) of 527 409 (40 637) new infections occurred with NPIs removed and a mean (SD) of 450 575 (32 716) new infections occurred with NPIs maintained. With NPIs removed, lower efficacy (50%) and higher coverage (75%) reduced infection risk by a greater magnitude than higher efficacy (90%) and lower coverage (25%) compared with the worst-case scenario (mean [SD] absolute risk reduction, 13% [1%] and 8% [1%], respectively).
Conclusions and relevance: Simulation outcomes suggest that removing NPIs while vaccines are distributed may result in substantial increases in infections, hospitalizations, and deaths. Furthermore, as NPIs are removed, higher vaccination coverage with less efficacious vaccines can contribute to a larger reduction in risk of SARS-CoV-2 infection compared with more efficacious vaccines at lower coverage. These findings highlight the need for well-resourced and coordinated efforts to achieve high vaccine coverage and continued adherence to NPIs before many prepandemic activities can be resumed.
Conflict of interest statement
Figures
Update of
-
The Joint Impact of COVID-19 Vaccination and Non-Pharmaceutical Interventions on Infections, Hospitalizations, and Mortality: An Agent-Based Simulation.medRxiv [Preprint]. 2021 Jan 10:2020.12.30.20248888. doi: 10.1101/2020.12.30.20248888. medRxiv. 2021. Update in: JAMA Netw Open. 2021 Jun 1;4(6):e2110782. doi: 10.1001/jamanetworkopen.2021.10782. PMID: 33442712 Free PMC article. Updated. Preprint.
Comment in
-
The Combined Effect of Vaccination and Nonpharmaceutical Public Health Interventions-Ending the COVID-19 Pandemic.JAMA Netw Open. 2021 Jun 1;4(6):e2111675. doi: 10.1001/jamanetworkopen.2021.11675. JAMA Netw Open. 2021. PMID: 34061205 No abstract available.
References
-
- US Department of Health and Human Services . Fact sheet: explaining Operation Warp Speed. Published June 6, 2020. Accessed September 17, 2020. https://www.hhs.gov/coronavirus/covid-19-vaccines/index.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

