Genetic Basis of Chromate Adaptation and the Role of the Pre-existing Genetic Divergence during an Experimental Evolution Study with Desulfovibrio vulgaris Populations
- PMID: 34061571
- PMCID: PMC8579811
- DOI: 10.1128/mSystems.00493-21
Genetic Basis of Chromate Adaptation and the Role of the Pre-existing Genetic Divergence during an Experimental Evolution Study with Desulfovibrio vulgaris Populations
Abstract
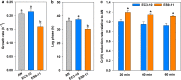
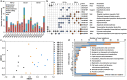
Hexavalent chromium [Cr(VI)] is a common environmental pollutant. However, little is known about the genetic basis of microbial evolution under Cr(VI) stress and the influence of the prior evolution histories on the subsequent evolution under Cr(VI) stress. In this study, Desulfovibrio vulgaris Hildenborough (DvH), a model sulfate-reducing bacterium, was experimentally evolved for 600 generations. By evolving the replicate populations of three genetically diverse DvH clones, including ancestor (AN, without prior experimental evolution history), non-stress-evolved EC3-10, and salt stress-evolved ES9-11, the contributions of adaptation, chance, and pre-existing genetic divergence to the evolution under Cr(VI) stress were able to be dissected. Significantly decreased lag phases under Cr(VI) stress were observed in most evolved populations, while increased Cr(VI) reduction rates were primarily observed in populations evolved from EC3-10 and ES9-11. The pre-existing genetic divergence in the starting clones showed strong influences on the changes in lag phases, growth rates, and Cr(VI) reduction rates. Additionally, the genomic mutation spectra in populations evolved from different starting clones were significantly different. A total of 14 newly mutated genes obtained mutations in at least two evolved populations, suggesting their importance in Cr(VI) adaptation. An in-frame deletion mutation of one of these genes, the chromate transporter gene DVU0426, demonstrated that it played an important role in Cr(VI) tolerance. Overall, our study identified potential key functional genes for Cr(VI) tolerance and demonstrated the important role of pre-existing genetic divergence in evolution under Cr(VI) stress conditions. IMPORTANCE Chromium is one of the most common heavy metal pollutants of soil and groundwater. The potential of Desulfovibrio vulgaris Hildenborough in heavy metal bioremediation such as Cr(VI) reduction was reported previously; however, experimental evidence of key functional genes involved in Cr(VI) resistance are largely unknown. Given the genetic divergence of microbial populations in nature, knowledge on how this divergence affects the microbial adaptation to a new environment such as Cr(VI) stress is very limited. Taking advantage of our previous study, three groups of genetically diverse D. vulgaris Hildenborough populations with or without prior experimental evolution histories were propagated under Cr(VI) stress for 600 generations. Whole-population genome resequencing of the evolved populations revealed the genomic changes underlying the improved Cr(VI) tolerance. The strong influence of the pre-existing genetic divergence in the starting clones on evolution under Cr(VI) stress conditions was demonstrated at both phenotypic and genetic levels.
Keywords: Desulfovibrio vulgaris; chromate stress; experimental evolution; genetic background.
Figures



References
-
- Zayed AM, Terry N. 2003. Chromium in the environment: factors affecting biological remediation. Plant Soil 249:139–156. doi: 10.1023/A:1022504826342. - DOI
-
- Thacker U, Parikh R, Shouche Y, Madamwar D. 2006. Hexavalent chromium reduction by Providencia sp. Process Biochem 41:1332–1337. doi: 10.1016/j.procbio.2006.01.006. - DOI
-
- Waldron HA. 1980. Metals in the environment. Academic Press Inc (London) Ltd, London, United Kingdom.
-
- Saranraj P, Sujitha D. 2013. Microbial bioremediation of chromium in tannery effluent: a review. Int J Microbiol Res 4:305–306.
-
- Palmisano A, Hazen T. 2003. Bioremediation of metals and radionuclides: what it is and how it works. Lawrence Berkeley National Laboratory, Berkeley, CA.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
