Increased Production of Outer Membrane Vesicles by Salmonella Interferes with Complement-Mediated Innate Immune Attack
- PMID: 34061589
- PMCID: PMC8262969
- DOI: 10.1128/mBio.00869-21
Increased Production of Outer Membrane Vesicles by Salmonella Interferes with Complement-Mediated Innate Immune Attack
Abstract
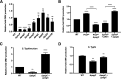
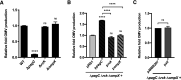
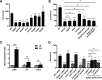
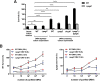
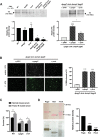
Bacterial outer membrane vesicles (OMVs) enriched with bioactive proteins, toxins, and virulence factors play a critical role in host-pathogen and microbial interactions. The two-component system PhoP-PhoQ (PhoPQ) of Salmonella enterica orchestrates the remodeling of outer membrane lipopolysaccharide (LPS) molecules and concomitantly upregulates OMV production. In this study, we document a novel use of nanoparticle tracking analysis to determine bacterial OMV size and number. Among the PhoPQ-activated genes tested, pagC expression had the most significant effect on the upregulation of OMV production. We provide the first evidence that PhoPQ-mediated upregulation of OMV production contributes to bacterial survival by interfering with complement activation. OMVs protected bacteria in a dose-dependent manner, and bacteria were highly susceptible to complement-mediated killing in their absence. OMVs from bacteria expressing PagC bound to complement component C3b in a dose-dependent manner and inactivated it by recruiting complement inhibitor Factor H. As we also found that Factor H binds to PagC, we propose that PagC interferes with complement-mediated killing of Salmonella in the following two steps: first by engaging Factor H, and second, through the production of PagC-enriched OMVs that divert and inactivate the complement away from the bacteria. Since PhoPQ activation occurs intracellularly, the resultant increase in PagC expression and OMV production is suggested to contribute to the local and systemic spread of Salmonella released from dying host cells that supports the infection of new cells. IMPORTANCE Bacterial outer membrane vesicles (OMVs) mediate critical bacterium-bacterium and host-microbial interactions that influence pathogenesis through multiple mechanisms, including the elicitation of inflammatory responses, delivery of virulence factors, and enhancement of biofilm formation. As such, there is a growing interest in understanding the underlying mechanisms of OMV production. Recent studies have revealed that OMV biogenesis is a finely tuned physiological process that requires structural organization and selective sorting of outer membrane components into the vesicles. In Salmonella, outer membrane remodeling and OMV production are tightly regulated by its PhoPQ system. In this study, we demonstrate that PhoPQ-regulated OMV production plays a significant role in defense against host innate immune attack. PhoPQ-activated PagC expression recruits the complement inhibitor Factor H and degrades the active C3 component of complement. Our results provide valuable insight into the combination of tools and environmental signals that Salmonella employs to evade complement-mediated lysis, thereby suggesting a strong evolutionary adaptation of this facultative intracellular pathogen to protect itself during its extracellular stage in the host.
Keywords: C3b; Factor H; PagC; PhoPQ; Rck; S. Typhimurium; Salmonella; complement resistance; outer membrane vesicles.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
