Potent Killing of Pseudomonas aeruginosa by an Antibody-Antibiotic Conjugate
- PMID: 34061593
- PMCID: PMC8262897
- DOI: 10.1128/mBio.00202-21
Potent Killing of Pseudomonas aeruginosa by an Antibody-Antibiotic Conjugate
Abstract
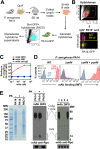
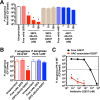
Pseudomonas aeruginosa causes life-threatening infections that are associated with antibiotic failure. Previously, we identified the antibiotic G2637, an analog of arylomycin, targeting bacterial type I signal peptidase, which has moderate potency against P. aeruginosa. We hypothesized that an antibody-antibiotic conjugate (AAC) could increase its activity by colocalizing P. aeruginosa bacteria with high local concentrations of G2637 antibiotic in the intracellular environment of phagocytes. Using a novel technology of screening for hybridomas recognizing intact bacteria, we identified monoclonal antibody 26F8, which binds to lipopolysaccharide O antigen on the surface of P. aeruginosa bacteria. This antibody was engineered to contain 6 cysteines and was conjugated to the G2637 antibiotic via a lysosomal cathepsin-cleavable linker, yielding a drug-to-antibody ratio of approximately 6. The resulting AAC delivered a high intracellular concentration of free G2637 upon phagocytosis of AAC-bound P. aeruginosa by macrophages, and potently cleared viable P. aeruginosa bacteria intracellularly. The molar concentration of AAC-associated G2637 antibiotic that resulted in elimination of bacteria inside macrophages was approximately 2 orders of magnitude lower than the concentration of free G2637 required to eliminate extracellular bacteria. This study demonstrates that an anti-P. aeruginosa AAC can locally concentrate antibiotic and kill P. aeruginosa inside phagocytes, providing additional therapeutic options for antibiotics that are moderately active or have an unfavorable pharmacokinetics or toxicity profile. IMPORTANCE Antibiotic treatment of life-threatening P. aeruginosa infections is associated with low clinical success, despite the availability of antibiotics that are active in standard microbiological in vitro assays, affirming the need for new therapeutic approaches. Antibiotics often fail in the preclinical stage due to insufficient efficacy against P. aeruginosa. One potential strategy is to enhance the local concentration of antibiotics with limited inherent anti-P. aeruginosa activity. This study presents proof of concept for an antibody-antibiotic conjugate, which releases a high local antibiotic concentration inside macrophages upon phagocytosis, resulting in potent intracellular killing of phagocytosed P. aeruginosa bacteria. This approach may provide new therapeutic options for antibiotics that are dose limited.
Keywords: Pseudomonas aeruginosa; antibiotics; antibody-antibiotic conjugate; macrophage.
Figures

References
-
- Thaden JT, Park LP, Maskarinec SA, Ruffin F, Fowler VG, van Duin D. 2017. Results from a 13-year prospective cohort study show increased mortality associated with bloodstream infections caused by Pseudomonas aeruginosa compared to other bacteria. Antimicrob Agents Chemother 61:e02671-16. - PMC - PubMed
-
- Planquette B, Timsit J-F, Misset BY, Schwebel C, Azoulay E, Adrie C, Vesin A, Jamali S, Zahar J-R, Allaouchiche B, Souweine B, Darmon M, Dumenil A-S, Goldgran-Toledano D, Mourvillier BH, Bédos J-P. 2013. Pseudomonas aeruginosa ventilator-associated pneumonia. Predictive factors of treatment failure. Am J Respir Crit Care Med 188:69–76. 10.1164/rccm.201210-1897OC. - DOI - PubMed
-
- Zilberberg MD, Shorr AF. 2013. Prevalence of multidrug-resistant pseudomonas aeruginosa and carbapenem-resistant enterobacteriaceae among specimens from hospitalized patients with pneumonia and bloodstream infections in the United States from 2000 to 2009: drug resistance in pneumonia and BSI. J Hosp Med 8:559–563. 10.1002/jhm.2080. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
