Obesity-Related Gut Microbiota Aggravates Alveolar Bone Destruction in Experimental Periodontitis through Elevation of Uric Acid
- PMID: 34061595
- PMCID: PMC8262938
- DOI: 10.1128/mBio.00771-21
Obesity-Related Gut Microbiota Aggravates Alveolar Bone Destruction in Experimental Periodontitis through Elevation of Uric Acid
Abstract
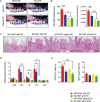
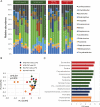
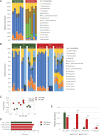
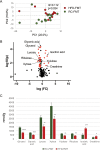
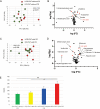
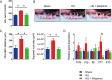
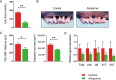
Obesity is a risk factor for periodontal disease (PD). Initiation and progression of PD are modulated by complex interactions between oral dysbiosis and host responses. Although obesity is associated with increased susceptibility to bacterial infection, the detailed mechanisms that connect obesity and susceptibility to PD remain elusive. Using fecal microbiota transplantation and a ligature-induced PD model, we demonstrated that gut dysbiosis-associated metabolites from high-fat diet (HFD)-fed mice worsen alveolar bone destruction. Fecal metabolomics revealed elevated purine degradation pathway activity in HFD-fed mice, and recipient mice had elevated levels of serum uric acid upon PD induction. Furthermore, PD induction caused more severe bone destruction in hyperuricemic than normouricemic mice, and the worsened bone destruction was completely abrogated by allopurinol, a xanthine oxidase inhibitor. Thus, obesity increases the risk of PD by increasing production of uric acid mediated by gut dysbiosis. IMPORTANCE Obesity is an epidemic health issue with a rapid increase worldwide. It increases the risk of various diseases, including periodontal disease, an oral chronic infectious disease. Although obesity increases susceptibility to bacterial infection, the precise biological mechanisms that link obesity and susceptibility to periodontal disease remain elusive. Using fecal microbial transplantation, experimental periodontitis, and metabolomics, our study demonstrates uric acid as a causative substance for greater aggravation of alveolar bone destruction in obesity-related periodontal disease. Gut microbiota from obese mice upregulated the purine degradation pathway, and the resulting elevation of serum uric acid promoted alveolar bone destruction. The effect of uric acid was confirmed by administration of allopurinol, an inhibitor of xanthine oxidase. Overall, our study provides new insights into the pathogenic mechanisms of obesity-associated periodontal disease and the development of new therapeutic options for the disease.
Keywords: gut microbiome; metabolomics; obesity; periodontal disease; uric acid.
Figures
References
-
- Kesavalu L, Bakthavatchalu V, Rahman MM, Su J, Raghu B, Dawson D, Fernandes G, Ebersole JL. 2007. Omega-3 fatty acid regulates inflammatory cytokine/mediator messenger RNA expression in Porphyromonas gingivalis-induced experimental periodontal disease. Oral Microbiol Immunol 22:232–239. doi: 10.1111/j.1399-302X.2007.00346.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
