Phylogeny Reveals Novel HipA-Homologous Kinase Families and Toxin-Antitoxin Gene Organizations
- PMID: 34061596
- PMCID: PMC8262856
- DOI: 10.1128/mBio.01058-21
Phylogeny Reveals Novel HipA-Homologous Kinase Families and Toxin-Antitoxin Gene Organizations
Abstract
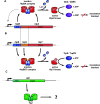
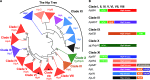
Toxin-antitoxin modules function in the genetic stability of mobile genetic elements, bacteriophage defense, and antibiotic tolerance. A gain-of-function mutation of the Escherichia coli K-12 hipBA module can induce antibiotic tolerance in a subpopulation of bacterial cells, a phenomenon known as persistence. HipA is a Ser/Thr kinase that phosphorylates and inactivates glutamyl tRNA synthetase, inhibiting cellular translation and inducing the stringent response. Additional characterized HipA homologues include HipT from pathogenic E. coli O127 and YjjJ of E. coli K-12, which are encoded by tricistronic hipBST and monocistronic operons, respectively. The apparent diversity of HipA homologues in bacterial genomes inspired us to investigate overall phylogeny. Here, we present a comprehensive phylogenetic analysis of the Hip kinases in bacteria and archaea that expands on this diversity by revealing seven novel kinase families. Kinases of one family, encoded by monocistronic operons, consist of an N-terminal core kinase domain, a HipS-like domain, and a HIRAN (HIP116 Rad5p N-terminal) domain. HIRAN domains bind single- or double-stranded DNA ends. Moreover, five types of bicistronic kinase operons encode putative antitoxins with HipS-HIRAN, HipS, γδ-resolvase, or Stl repressor-like domains. Finally, our analysis indicates that reversion of hipBA gene order happened independently several times during evolution. IMPORTANCE Bacterial multidrug tolerance and persistence are problems of increasing scientific and medical significance. The first gene discovered to confer persistence was hipA, encoding the kinase toxin of the hipBA toxin-antitoxin (TA) module of E. coli. HipA-homologous kinases phosphorylate and thereby inactivate specific tRNA synthetases, thus inhibiting protein translation and cell proliferation. Here, we present a comprehensive phylogenetic analysis of bacterial Hip kinases and discover seven new families with novel operon structures and domains. Overall, Hip kinases are encoded by TA modules with at least 10 different genetic organizations, 7 of which have not been described before. These results open up exciting avenues for the experimental analysis of the superfamily of Hip kinases.
Keywords: GltX; HIRAN; HipB; HipS; HipT; Stl; TrpS; high persister A; kinase.
Figures



References
-
- Hiraga S, Ogura T, Mori H, Tanaka M. 1985. Mechanisms essential for stable inheritance of mini-F plasmid. Basic Life Sci 30:469–487. - PubMed
-
- Gerdes K, Bech FW, Jorgensen ST, Lobner-Olesen A, Rasmussen PB, Atlung T, Boe L, Karlstrom O, Molin S, von Meyenburg K. 1986. Mechanism of postsegregational killing by the hok gene product of the parB system of plasmid R1 and its homology with the relF gene product of the E. coli relB operon. EMBO J 5:2023–2029. doi: 10.1002/j.1460-2075.1986.tb04459.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

