A Structural Model for the Ligand Binding of Pneumococcal Serotype 3 Capsular Polysaccharide-Specific Protective Antibodies
- PMID: 34061603
- PMCID: PMC8262990
- DOI: 10.1128/mBio.00800-21
A Structural Model for the Ligand Binding of Pneumococcal Serotype 3 Capsular Polysaccharide-Specific Protective Antibodies
Abstract
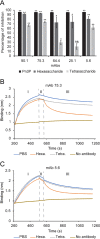
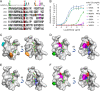
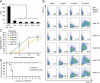
Capsular polysaccharides (CPSs) are major virulence factors that decorate the surfaces of many human bacterial pathogens. In their pure form or as glycoconjugate vaccines, CPSs are extensively used in vaccines deployed in clinical practice worldwide. However, our understanding of the structural requirements for interactions between CPSs and antibodies is limited. A longstanding model based on comprehensive observations of antibody repertoires binding to CPSs is that antibodies expressing heavy chain variable gene family 3 (VH3) predominate in these binding interactions in humans and VH3 homologs in mice. Toward understanding this highly conserved interaction, we generated a panel of mouse monoclonal antibodies (MAb) against Streptococcus pneumoniae serotype 3 CPS, determined an X-ray crystal structure of a protective MAb in complex with a hexasaccharide derived from enzymatic hydrolysis of the polysaccharide, and elucidated the structural requirements for this binding interaction. The crystal structure revealed a binding pocket containing aromatic side chains, suggesting the importance of hydrophobicity in the interaction. Through mutational analysis, we determined the amino acids that are critical in carbohydrate binding. Through elucidating the structural and functional properties of a panel of murine MAbs, we offer an explanation for the predominant use of the human VH3 gene family in antibodies against CPSs with implications in knowledge-based vaccine design. IMPORTANCE Infectious diseases caused by pathogenic bacteria are a major threat to human health. Capsular polysaccharides (CPSs) of many pathogenic bacteria have been used as the main components of glycoconjugate vaccines against bacterial diseases in clinical practice worldwide, with various degrees of success. Immunization with a glycoconjugate vaccine elicits T cell help for B cells that produce IgG antibodies to the CPS. Thus, it is important to develop an in-depth understanding of the interactions of carbohydrate epitopes with the antibodies. Structural characterization of the ligand binding of polysaccharide-specific antibodies laid out in this study may have fundamental biological implications for our comprehension of how the humoral immune system recognizes polysaccharide antigens, and in future knowledge-based vaccine design.
Keywords: Streptococcus pneumoniae; VH3 gene family; capsular polysaccharide; carbohydrate antigens; conjugate vaccines; glycoconjugate vaccine; monoclonal antibodies; monoclonal antibody.
Figures
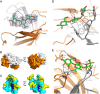
Similar articles
-
Isolation and Characterization of Human Monoclonal Antibodies to Pneumococcal Capsular Polysaccharide 3.Microbiol Spectr. 2021 Dec 22;9(3):e0144621. doi: 10.1128/Spectrum.01446-21. Epub 2021 Nov 10. Microbiol Spectr. 2021. PMID: 34756090 Free PMC article.
-
Deciphering Antigenic Determinants of Streptococcus pneumoniae Serotype 4 Capsular Polysaccharide using Synthetic Oligosaccharides.ACS Chem Biol. 2016 Feb 19;11(2):335-44. doi: 10.1021/acschembio.5b00768. Epub 2016 Jan 4. ACS Chem Biol. 2016. PMID: 26674834
-
A semisynthetic Streptococcus pneumoniae serotype 8 glycoconjugate vaccine.Sci Transl Med. 2017 Mar 8;9(380):eaaf5347. doi: 10.1126/scitranslmed.aaf5347. Sci Transl Med. 2017. PMID: 28275152 Free PMC article.
-
Immunogenicity differences of a 15-valent pneumococcal polysaccharide conjugate vaccine (PCV15) based on vaccine dose, route of immunization and mouse strain.Vaccine. 2017 Feb 7;35(6):865-872. doi: 10.1016/j.vaccine.2016.12.055. Epub 2017 Jan 10. Vaccine. 2017. PMID: 28087148 Review.
-
Pneumococcal Capsules and Their Types: Past, Present, and Future.Clin Microbiol Rev. 2015 Jul;28(3):871-99. doi: 10.1128/CMR.00024-15. Clin Microbiol Rev. 2015. PMID: 26085553 Free PMC article. Review.
Cited by
-
Pneumococcal Surface Proteins as Virulence Factors, Immunogens, and Conserved Vaccine Targets.Front Cell Infect Microbiol. 2022 May 12;12:832254. doi: 10.3389/fcimb.2022.832254. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35646747 Free PMC article. Review.
-
Isolation and Characterization of Human Monoclonal Antibodies to Pneumococcal Capsular Polysaccharide 3.Microbiol Spectr. 2021 Dec 22;9(3):e0144621. doi: 10.1128/Spectrum.01446-21. Epub 2021 Nov 10. Microbiol Spectr. 2021. PMID: 34756090 Free PMC article.
-
Structural diversity among Acinetobacter baumannii K-antigens and its implication in the in silico serotyping.Front Microbiol. 2023 Jun 21;14:1191542. doi: 10.3389/fmicb.2023.1191542. eCollection 2023. Front Microbiol. 2023. PMID: 37415807 Free PMC article.
-
Synergistic Protection against Secondary Pneumococcal Infection by Human Monoclonal Antibodies Targeting Distinct Epitopes.J Immunol. 2023 Jan 1;210(1):50-60. doi: 10.4049/jimmunol.2200349. Epub 2022 Nov 9. J Immunol. 2023. PMID: 36351696 Free PMC article.
-
A Multidisciplinary Structural Approach to the Identification of the Haemophilus influenzae Type b Capsular Polysaccharide Protective Epitope.ACS Cent Sci. 2024 Feb 22;10(5):978-987. doi: 10.1021/acscentsci.3c01515. eCollection 2024 May 22. ACS Cent Sci. 2024. PMID: 38799664 Free PMC article.
References
-
- Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger WD, Frasch CE, Stephens DS, Feavers I, Frosch M, Parkhill J, Vogel U, Quail MA, Bentley SD, Maiden MC. 2013. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis 19:566–573. doi:10.3201/eid1904.111799. - DOI - PMC - PubMed
-
- Pickard D, Wain J, Baker S, Line A, Chohan S, Fookes M, Barron A, Gaora PO, Chabalgoity JA, Thanky N, Scholes C, Thomson N, Quail M, Parkhill J, Dougan G. 2003. Composition, acquisition, and distribution of the Vi exopolysaccharide-encoding Salmonella enterica pathogenicity island SPI-7. J Bacteriol 185:5055–5065. doi:10.1128/jb.185.17.5055-5065.2003. - DOI - PMC - PubMed
-
- Zhensong W, Jing-Ren Z. 2015. Bacterial capsules, p 33–53. In Tang Y-W, Liu D, Schwartzman J, Sussman M, Poxton I (ed), Molecular Medical Microbiology, 2nd ed. Academic Press, Elsevier, Ltd.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
