Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1
- PMID: 34061776
- PMCID: PMC8279584
- DOI: 10.1172/JCI150867
Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1
Abstract
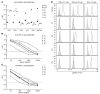
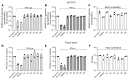
Autoantibodies against IFN-α and IFN-ω (type I IFNs) were recently reported as causative for severe COVID-19 in the general population. Autoantibodies against IFN-α and IFN-ω are present in almost all patients with autoimmune polyendocrine syndrome type 1 (APS-1) caused by biallelic deleterious or heterozygous dominant mutations in AIRE. We therefore hypothesized that autoantibodies against type I IFNs also predispose patients with APS-1 to severe COVID-19. We prospectively studied 6 patients with APS-1 between April 1, 2020 and April 1, 2021. Biobanked pre-COVID-19 sera of APS-1 subjects were tested for neutralizing autoantibodies against IFN-α and IFN-ω. The ability of the patients' sera to block recombinant human IFN-α and IFN-ω was assessed by assays quantifying phosphorylation of signal transducer and activator of transcription 1 (STAT1) as well as infection-based IFN-neutralization assays. We describe 4 patients with APS-1 and preexisting high titers of neutralizing autoantibodies against IFN-α and IFN-ω who contracted SARS-CoV-2, yet developed only mild symptoms of COVID-19. None of the patients developed dyspnea, oxygen requirement, or high temperature. All infected patients with APS-1 were females and younger than 26 years of age. Clinical penetrance of neutralizing autoantibodies against type I IFNs for severe COVID-19 is not complete.
Keywords: COVID-19; Immunology; Innate immunity.
Conflict of interest statement
Figures
Similar articles
-
Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1.J Exp Med. 2021 Jul 5;218(7):e20210554. doi: 10.1084/jem.20210554. J Exp Med. 2021. PMID: 33890986 Free PMC article.
-
Assessment of autoantibodies to interferon-ω in patients with autoimmune polyendocrine syndrome type 1: using a new immunoprecipitation assay.Clin Chem Lab Med. 2017 Jun 27;55(7):1003-1012. doi: 10.1515/cclm-2016-0615. Clin Chem Lab Med. 2017. PMID: 28099118
-
Autoimmune polyendocrine syndrome type 1 in Norway: phenotypic variation, autoantibodies, and novel mutations in the autoimmune regulator gene.J Clin Endocrinol Metab. 2007 Feb;92(2):595-603. doi: 10.1210/jc.2006-1873. Epub 2006 Nov 21. J Clin Endocrinol Metab. 2007. PMID: 17118990
-
Candidiasis in patients with APS-1: low IL-17, high IFN-γ, or both?Curr Opin Immunol. 2021 Oct;72:318-323. doi: 10.1016/j.coi.2021.08.001. Epub 2021 Aug 26. Curr Opin Immunol. 2021. PMID: 34455138 Free PMC article. Review.
-
Anti-Interferon Autoantibodies in Adult-Onset Immunodeficiency Syndrome and Severe COVID-19 Infection.Front Immunol. 2021 Dec 22;12:788368. doi: 10.3389/fimmu.2021.788368. eCollection 2021. Front Immunol. 2021. PMID: 35003106 Free PMC article. Review.
Cited by
-
Immune-epithelial cell cross-talk enhances antiviral responsiveness to SARS-CoV-2 in children.EMBO Rep. 2023 Dec 6;24(12):e57912. doi: 10.15252/embr.202357912. Epub 2023 Oct 11. EMBO Rep. 2023. PMID: 37818799 Free PMC article.
-
Virus Infection and Systemic Inflammation: Lessons Learnt from COVID-19 and Beyond.Cells. 2022 Jul 14;11(14):2198. doi: 10.3390/cells11142198. Cells. 2022. PMID: 35883640 Free PMC article. Review.
-
Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy.Front Pediatr. 2021 Nov 1;9:723532. doi: 10.3389/fped.2021.723532. eCollection 2021. Front Pediatr. 2021. PMID: 34790633 Free PMC article. Review.
-
B Cells and Autoantibodies in AIRE Deficiency.Biomedicines. 2021 Sep 21;9(9):1274. doi: 10.3390/biomedicines9091274. Biomedicines. 2021. PMID: 34572460 Free PMC article. Review.
-
Vaccination prevents severe COVID-19 outcome in patients with neutralizing type 1 interferon autoantibodies.iScience. 2023 Jul 21;26(7):107084. doi: 10.1016/j.isci.2023.107084. Epub 2023 Jun 9. iScience. 2023. PMID: 37346050 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

