High-throughput framework for genetic analyses of adverse drug reactions using electronic health records
- PMID: 34061827
- PMCID: PMC8195357
- DOI: 10.1371/journal.pgen.1009593
High-throughput framework for genetic analyses of adverse drug reactions using electronic health records
Abstract
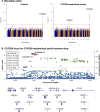
Understanding the contribution of genetic variation to drug response can improve the delivery of precision medicine. However, genome-wide association studies (GWAS) for drug response are uncommon and are often hindered by small sample sizes. We present a high-throughput framework to efficiently identify eligible patients for genetic studies of adverse drug reactions (ADRs) using "drug allergy" labels from electronic health records (EHRs). As a proof-of-concept, we conducted GWAS for ADRs to 14 common drug/drug groups with 81,739 individuals from Vanderbilt University Medical Center's BioVU DNA Biobank. We identified 7 genetic loci associated with ADRs at P < 5 × 10-8, including known genetic associations such as CYP2D6 and OPRM1 for CYP2D6-metabolized opioid ADR. Additional expression quantitative trait loci and phenome-wide association analyses added evidence to the observed associations. Our high-throughput framework is both scalable and portable, enabling impactful pharmacogenomic research to improve precision medicine.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

