Growth rules for the repair of Asynchronous Irregular neuronal networks after peripheral lesions
- PMID: 34061830
- PMCID: PMC8195387
- DOI: 10.1371/journal.pcbi.1008996
Growth rules for the repair of Asynchronous Irregular neuronal networks after peripheral lesions
Abstract
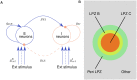
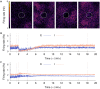
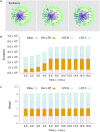
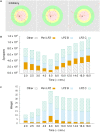
Several homeostatic mechanisms enable the brain to maintain desired levels of neuronal activity. One of these, homeostatic structural plasticity, has been reported to restore activity in networks disrupted by peripheral lesions by altering their neuronal connectivity. While multiple lesion experiments have studied the changes in neurite morphology that underlie modifications of synapses in these networks, the underlying mechanisms that drive these changes are yet to be explained. Evidence suggests that neuronal activity modulates neurite morphology and may stimulate neurites to selective sprout or retract to restore network activity levels. We developed a new spiking network model of peripheral lesioning and accurately reproduced the characteristics of network repair after deafferentation that are reported in experiments to study the activity dependent growth regimes of neurites. To ensure that our simulations closely resemble the behaviour of networks in the brain, we model deafferentation in a biologically realistic balanced network model that exhibits low frequency Asynchronous Irregular (AI) activity as observed in cerebral cortex. Our simulation results indicate that the re-establishment of activity in neurons both within and outside the deprived region, the Lesion Projection Zone (LPZ), requires opposite activity dependent growth rules for excitatory and inhibitory post-synaptic elements. Analysis of these growth regimes indicates that they also contribute to the maintenance of activity levels in individual neurons. Furthermore, in our model, the directional formation of synapses that is observed in experiments requires that pre-synaptic excitatory and inhibitory elements also follow opposite growth rules. Lastly, we observe that our proposed structural plasticity growth rules and the inhibitory synaptic plasticity mechanism that also balances our AI network both contribute to the restoration of the network to pre-deafferentation stable activity levels.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







Similar articles
-
Distinct Heterosynaptic Plasticity in Fast Spiking and Non-Fast-Spiking Inhibitory Neurons in Rat Visual Cortex.J Neurosci. 2019 Aug 28;39(35):6865-6878. doi: 10.1523/JNEUROSCI.3039-18.2019. Epub 2019 Jul 12. J Neurosci. 2019. PMID: 31300522 Free PMC article.
-
Balanced excitation and inhibition are required for high-capacity, noise-robust neuronal selectivity.Proc Natl Acad Sci U S A. 2017 Oct 31;114(44):E9366-E9375. doi: 10.1073/pnas.1705841114. Epub 2017 Oct 17. Proc Natl Acad Sci U S A. 2017. PMID: 29042519 Free PMC article.
-
Transition from Asynchronous to Oscillatory Dynamics in Balanced Spiking Networks with Instantaneous Synapses.Phys Rev Lett. 2018 Sep 21;121(12):128301. doi: 10.1103/PhysRevLett.121.128301. Phys Rev Lett. 2018. PMID: 30296134
-
[Acquiring new information in a neuronal network: from Hebb's concept to homeostatic plasticity].J Soc Biol. 2008;202(2):143-60. doi: 10.1051/jbio:2008018. Epub 2008 Jun 13. J Soc Biol. 2008. PMID: 18547512 Review. French.
-
Homeostatic plasticity in neural development.Neural Dev. 2018 Jun 1;13(1):9. doi: 10.1186/s13064-018-0105-x. Neural Dev. 2018. PMID: 29855353 Free PMC article. Review.
Cited by
-
Nutrition Strategies Promoting Healthy Aging: From Improvement of Cardiovascular and Brain Health to Prevention of Age-Associated Diseases.Nutrients. 2022 Dec 22;15(1):47. doi: 10.3390/nu15010047. Nutrients. 2022. PMID: 36615705 Free PMC article. Review.
-
Resolving inconsistent effects of tDCS on learning using a homeostatic structural plasticity model.Front Netw Physiol. 2025 Jul 7;5:1565802. doi: 10.3389/fnetp.2025.1565802. eCollection 2025. Front Netw Physiol. 2025. PMID: 40692813 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources

