Global economic costs due to vivax malaria and the potential impact of its radical cure: A modelling study
- PMID: 34061843
- PMCID: PMC8168905
- DOI: 10.1371/journal.pmed.1003614
Global economic costs due to vivax malaria and the potential impact of its radical cure: A modelling study
Abstract
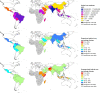
Background: In 2017, an estimated 14 million cases of Plasmodium vivax malaria were reported from Asia, Central and South America, and the Horn of Africa. The clinical burden of vivax malaria is largely driven by its ability to form dormant liver stages (hypnozoites) that can reactivate to cause recurrent episodes of malaria. Elimination of both the blood and liver stages of the parasites ("radical cure") is required to achieve a sustained clinical response and prevent ongoing transmission of the parasite. Novel treatment options and point-of-care diagnostics are now available to ensure that radical cure can be administered safely and effectively. We quantified the global economic cost of vivax malaria and estimated the potential cost benefit of a policy of radical cure after testing patients for glucose-6-phosphate dehydrogenase (G6PD) deficiency.
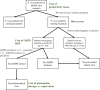
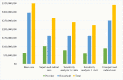
Methods and findings: Estimates of the healthcare provider and household costs due to vivax malaria were collated and combined with national case estimates for 44 endemic countries in 2017. These provider and household costs were compared with those that would be incurred under 2 scenarios for radical cure following G6PD screening: (1) complete adherence following daily supervised primaquine therapy and (2) unsupervised treatment with an assumed 40% effectiveness. A probabilistic sensitivity analysis generated credible intervals (CrIs) for the estimates. Globally, the annual cost of vivax malaria was US$359 million (95% CrI: US$222 to 563 million), attributable to 14.2 million cases of vivax malaria in 2017. From a societal perspective, adopting a policy of G6PD deficiency screening and supervision of primaquine to all eligible patients would prevent 6.1 million cases and reduce the global cost of vivax malaria to US$266 million (95% CrI: US$161 to 415 million), although healthcare provider costs would increase by US$39 million. If perfect adherence could be achieved with a single visit, then the global cost would fall further to US$225 million, equivalent to $135 million in cost savings from the baseline global costs. A policy of unsupervised primaquine reduced the cost to US$342 million (95% CrI: US$209 to 532 million) while preventing 2.1 million cases. Limitations of the study include partial availability of country-level cost data and parameter uncertainty for the proportion of patients prescribed primaquine, patient adherence to a full course of primaquine, and effectiveness of primaquine when unsupervised.
Conclusions: Our modelling study highlights a substantial global economic burden of vivax malaria that could be reduced through investment in safe and effective radical cure achieved by routine screening for G6PD deficiency and supervision of treatment. Novel, low-cost interventions for improving adherence to primaquine to ensure effective radical cure and widespread access to screening for G6PD deficiency will be critical to achieving the timely global elimination of P. vivax.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Using G6PD tests to enable the safe treatment of Plasmodium vivax infections with primaquine on the Thailand-Myanmar border: A cost-effectiveness analysis.PLoS Negl Trop Dis. 2017 May 24;11(5):e0005602. doi: 10.1371/journal.pntd.0005602. eCollection 2017 May. PLoS Negl Trop Dis. 2017. PMID: 28542194 Free PMC article.
-
High daily dose Short COurse PrimaquinE after G6PD testing for the radical cure of Plasmodium vivax malaria in Indonesia and Papua New Guinea: the SCOPE implementation study protocol.BMC Infect Dis. 2025 Jul 16;25(1):922. doi: 10.1186/s12879-025-11109-9. BMC Infect Dis. 2025. PMID: 40670924 Free PMC article.
-
Cost-effectiveness analysis of G6PD diagnostic test for Plasmodium vivax radical cure in Lao PDR: An economic modelling study.PLoS One. 2022 Apr 25;17(4):e0267193. doi: 10.1371/journal.pone.0267193. eCollection 2022. PLoS One. 2022. PMID: 35468145 Free PMC article.
-
Use of primaquine and glucose-6-phosphate dehydrogenase deficiency testing: Divergent policies and practices in malaria endemic countries.PLoS Negl Trop Dis. 2018 Apr 19;12(4):e0006230. doi: 10.1371/journal.pntd.0006230. eCollection 2018 Apr. PLoS Negl Trop Dis. 2018. PMID: 29672516 Free PMC article. Review.
-
Primaquine alternative dosing schedules for preventing malaria relapse in people with Plasmodium vivax.Cochrane Database Syst Rev. 2020 Aug 19;8:CD012656. doi: 10.1002/14651858.CD012656.pub3. Cochrane Database Syst Rev. 2020. PMID: 32816320
Cited by
-
Targeting anemia-induced CD71+ reticulocytes protects mice from Plasmodium infection.Infect Immun. 2025 Aug 12;93(8):e0009325. doi: 10.1128/iai.00093-25. Epub 2025 Jul 1. Infect Immun. 2025. PMID: 40590713 Free PMC article.
-
Mathematical models of Plasmodium vivax transmission: A scoping review.PLoS Comput Biol. 2024 Mar 14;20(3):e1011931. doi: 10.1371/journal.pcbi.1011931. eCollection 2024 Mar. PLoS Comput Biol. 2024. PMID: 38483975 Free PMC article.
-
Web-based models to inform health policy: A scoping review.Health Res Policy Syst. 2025 Aug 4;23(1):99. doi: 10.1186/s12961-025-01367-z. Health Res Policy Syst. 2025. PMID: 40760496 Free PMC article. Review.
-
Assessing the Operational Feasibility of Integrating Point-of-Care G6PD Testing into Plasmodium vivax Malaria Management in Vietnam.Pathogens. 2023 May 8;12(5):689. doi: 10.3390/pathogens12050689. Pathogens. 2023. PMID: 37242359 Free PMC article.
-
Malaria morbidity, mortality and associated costs in Indonesia: analysis of the National Health Insurance claim dataset.BMJ Glob Health. 2025 May 12;10(5):e018255. doi: 10.1136/bmjgh-2024-018255. BMJ Glob Health. 2025. PMID: 40355266 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

