The effect of prior long-term recellularization with keratocytes of decellularized porcine corneas implanted in a rabbit anterior lamellar keratoplasty model
- PMID: 34061862
- PMCID: PMC8168847
- DOI: 10.1371/journal.pone.0245406
The effect of prior long-term recellularization with keratocytes of decellularized porcine corneas implanted in a rabbit anterior lamellar keratoplasty model
Abstract
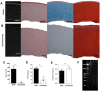
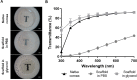
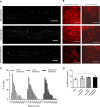
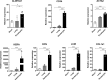
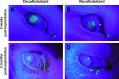
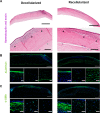
Decellularized porcine corneal scaffolds are a potential alternative to human cornea for keratoplasty. Although clinical trials have reported promising results, there can be corneal haze or scar tissue. Here, we examined if recellularizing the scaffolds with human keratocytes would result in a better outcome. Scaffolds were prepared that retained little DNA (14.89 ± 5.56 ng/mg) and demonstrated a lack of cytotoxicity by in vitro. The scaffolds were recellularized using human corneal stromal cells and cultured for between 14 in serum-supplemented media followed by a further 14 days in either serum free or serum-supplemented media. All groups showed full-depth cell penetration after 14 days. When serum was present, staining for ALDH3A1 remained weak but after serum-free culture, staining was brighter and the keratocytes adopted a native dendritic morphology with an increase (p < 0.05) of keratocan, decorin, lumican and CD34 gene expression. A rabbit anterior lamellar keratoplasty model was used to compare implanting a 250 μm thick decellularized lenticule against one that had been recellularized with human stromal cells after serum-free culture. In both groups, host rabbit epithelium covered the implants, but transparency was not restored after 3 months. Post-mortem histology showed under the epithelium, a less-compact collagen layer, which appeared to be a regenerating zone with some α-SMA staining, indicating fibrotic cells. In the posterior scaffold, ALDH1A1 staining was present in all the acellular scaffold, but in only one of the recellularized lenticules. Since there was little difference between acellular and cell-seeded scaffolds in our in vivo study, future scaffold development should use acellular controls to determine if cells are necessary.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
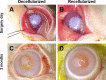
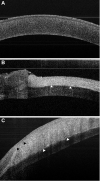

Similar articles
-
Engineering a Corneal Stromal Equivalent Using a Novel Multilayered Fabrication Assembly Technique.Tissue Eng Part A. 2020 Oct;26(19-20):1030-1041. doi: 10.1089/ten.TEA.2020.0019. Epub 2020 Jun 25. Tissue Eng Part A. 2020. PMID: 32368948 Free PMC article.
-
Acellular human corneal matrix sheets seeded with human adipose-derived mesenchymal stem cells integrate functionally in an experimental animal model.Exp Eye Res. 2015 Mar;132:91-100. doi: 10.1016/j.exer.2015.01.020. Epub 2015 Jan 24. Exp Eye Res. 2015. PMID: 25625506
-
Reconstruction of a tissue-engineered cornea with porcine corneal acellular matrix as the scaffold.Cells Tissues Organs. 2010;191(3):193-202. doi: 10.1159/000235680. Epub 2009 Aug 19. Cells Tissues Organs. 2010. PMID: 19690400
-
[Transplantation of corneal endothelial cells].Nippon Ganka Gakkai Zasshi. 2002 Dec;106(12):805-35; discussion 836. Nippon Ganka Gakkai Zasshi. 2002. PMID: 12610838 Review. Japanese.
-
Human SMILE-Derived Stromal Lenticule Scaffold for Regenerative Therapy: Review and Perspectives.Int J Mol Sci. 2022 Jul 19;23(14):7967. doi: 10.3390/ijms23147967. Int J Mol Sci. 2022. PMID: 35887309 Free PMC article. Review.
Cited by
-
Induction of Corneal Epithelial Differentiation of Induced Pluripotent and Orbital Fat-Derived Stem Cells Seeded on Decellularized Human Corneas.Stem Cell Rev Rep. 2022 Oct;18(7):2522-2534. doi: 10.1007/s12015-022-10356-6. Epub 2022 Mar 5. Stem Cell Rev Rep. 2022. PMID: 35247143
-
On the Mechanical Roles of Glycosaminoglycans in the Tensile Properties of Porcine Corneal Stroma.Invest Ophthalmol Vis Sci. 2023 Apr 3;64(4):3. doi: 10.1167/iovs.64.4.3. Invest Ophthalmol Vis Sci. 2023. PMID: 37014650 Free PMC article.
-
Applications of SMILE-extracted lenticules in ophthalmology.Int J Ophthalmol. 2024 Jan 18;17(1):173-187. doi: 10.18240/ijo.2024.01.23. eCollection 2024. Int J Ophthalmol. 2024. PMID: 38239948 Free PMC article. Review.
-
The Impact of the Extracellular Matrix Environment on Sost Expression by the MLO-Y4 Osteocyte Cell Line.Bioengineering (Basel). 2022 Jan 13;9(1):35. doi: 10.3390/bioengineering9010035. Bioengineering (Basel). 2022. PMID: 35049744 Free PMC article.
References
-
- Alió Del Barrio JL, El Zarif M, Azaar A, Makdissy N, Khalil C, Harb W, et al. Corneal stroma enhancement with decellularized stromal laminas with or without stem cell recellularization for advanced keratoconus. Am J Ophthalmol. 2018;186:47–58. Epub 2017/11/07. 10.1016/j.ajo.2017.10.026 . - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

