Division of labor of Y-family polymerases in translesion-DNA synthesis for distinct types of DNA damage
- PMID: 34061890
- PMCID: PMC8168857
- DOI: 10.1371/journal.pone.0252587
Division of labor of Y-family polymerases in translesion-DNA synthesis for distinct types of DNA damage
Abstract
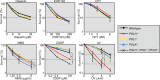
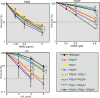
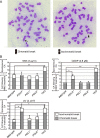
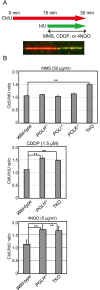
Living organisms are continuously under threat from a vast array of DNA-damaging agents, which impact genome DNA. DNA replication machinery stalls at damaged template DNA. The stalled replication fork is restarted via bypass replication by translesion DNA-synthesis polymerases, including the Y-family polymerases Polη, Polι, and Polκ, which possess the ability to incorporate nucleotides opposite the damaged template. To investigate the division of labor among these polymerases in vivo, we generated POLη-/-, POLι-/-, POLκ-/-, double knockout (KO), and triple knockout (TKO) mutants in all combinations from human TK6 cells. TKO cells exhibited a hypersensitivity to ultraviolet (UV), cisplatin (CDDP), and methyl methanesulfonate (MMS), confirming the pivotal role played by these polymerases in bypass replication of damaged template DNA. POLη-/- cells, but not POLι-/- or POLκ-/- cells, showed a strong sensitivity to UV and CDDP, while TKO cells showed a slightly higher sensitivity to UV and CDDP than did POLη-/- cells. On the other hand, TKO cells, but not all single KO cells, exhibited a significantly higher sensitivity to MMS than did wild-type cells. Consistently, DNA-fiber assay revealed that Polη plays a crucial role in bypassing lesions caused by UV-mimetic agent 4-nitroquinoline-1-oxide and CDDP, while all three polymerases play complementary roles in bypassing MMS-induced damage. Our findings indicate that the three Y-family polymerases play distinctly different roles in bypass replication, according to the type of DNA damage generated on the template strand.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Hypersensitivity of mouse embryonic fibroblast cells defective for DNA polymerases η, ι and κ to various genotoxic compounds: Its potential for application in chemical genotoxic screening.DNA Repair (Amst). 2018 Jan;61:76-85. doi: 10.1016/j.dnarep.2017.11.006. Epub 2017 Nov 26. DNA Repair (Amst). 2018. PMID: 29247828
-
Localization of DNA polymerases eta and iota to the replication machinery is tightly co-ordinated in human cells.EMBO J. 2002 Nov 15;21(22):6246-56. doi: 10.1093/emboj/cdf618. EMBO J. 2002. Corrected and republished in: EMBO J. 2003 Mar 3;22(5):1223-33. doi: 10.1093/emboj/cdf618. PMID: 12426396 Free PMC article. Corrected and republished.
-
Localization of DNA polymerases eta and iota to the replication machinery is tightly co-ordinated in human cells.EMBO J. 2003 Mar 3;22(5):1223-33. doi: 10.1093/emboj/cdf618. EMBO J. 2003. PMID: 12606586 Free PMC article.
-
poliota-dependent lesion bypass in vitro.Mutat Res. 2002 Dec 29;510(1-2):9-22. doi: 10.1016/s0027-5107(02)00248-8. Mutat Res. 2002. PMID: 12459439 Review.
-
The roles of DNA polymerase ζ and the Y family DNA polymerases in promoting or preventing genome instability.Mutat Res. 2013 Mar-Apr;743-744:97-110. doi: 10.1016/j.mrfmmm.2012.11.002. Epub 2012 Nov 26. Mutat Res. 2013. PMID: 23195997 Free PMC article. Review.
Cited by
-
DIFFERENTIAL ROLES OF RAD18 IN REPRESSING CARCINOGEN- AND ONCOGENE-DRIVEN MUTAGENESIS IN VIVO.bioRxiv [Preprint]. 2025 Jul 4:2025.06.30.662411. doi: 10.1101/2025.06.30.662411. bioRxiv. 2025. PMID: 40631250 Free PMC article. Preprint.
-
The flap endonuclease-1 promotes cellular tolerance to a chain-terminating nucleoside analog, alovudine, by counteracting the toxic effect of 53BP1.Nucleic Acids Res. 2025 Jul 8;53(13):gkaf617. doi: 10.1093/nar/gkaf617. Nucleic Acids Res. 2025. PMID: 40685548 Free PMC article.
-
A New Drug Discovery Platform: Application to DNA Polymerase Eta and Apurinic/Apyrimidinic Endonuclease 1.Int J Mol Sci. 2023 Nov 23;24(23):16637. doi: 10.3390/ijms242316637. Int J Mol Sci. 2023. PMID: 38068959 Free PMC article.
-
Beyond the Lesion: Back to High Fidelity DNA Synthesis.Front Mol Biosci. 2022 Jan 5;8:811540. doi: 10.3389/fmolb.2021.811540. eCollection 2021. Front Mol Biosci. 2022. PMID: 35071328 Free PMC article. Review.
-
PRIMPOL ensures robust handoff between on-the-fly and post-replicative DNA lesion bypass.Nucleic Acids Res. 2024 Jan 11;52(1):243-258. doi: 10.1093/nar/gkad1054. Nucleic Acids Res. 2024. PMID: 37971291 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

