Effect of NAD+ boosting on kidney ischemia-reperfusion injury
- PMID: 34061900
- PMCID: PMC8168908
- DOI: 10.1371/journal.pone.0252554
Effect of NAD+ boosting on kidney ischemia-reperfusion injury
Abstract
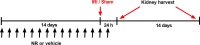
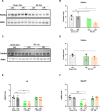
Acute kidney injury (AKI) is associated with a very high mortality and an increased risk for progression to chronic kidney disease (CKD). Ischemia-reperfusion injury (IRI) is a model for AKI, which results in tubular damage, dysfunction of the mitochondria and autophagy, and in decreased cellular nicotinamide adenine dinucleotide (NAD+) with progressing fibrosis resulting in CKD. NAD+ is a co-enzyme for several proteins, including the NAD+ dependent sirtuins. NAD+ augmentation, e.g. by use of its precursor nicotinamide riboside (NR), improves mitochondrial homeostasis and organismal metabolism in many species. In the present investigation the effects of prophylactic administration of NR on IRI-induced AKI were studied in the rat. Bilateral IRI reduced kidney tissue NAD+, caused tubular damage, reduced α-Klotho (klotho), and altered autophagy flux. AKI initiated progression to CKD, as shown by induced profibrotic Periostin (postn) and Inhibin subunit beta-A, (activin A / Inhba), both 24 hours and 14 days after surgery. NR restored tissue NAD+ to that of the sham group, increased autophagy (reduced p62) and sirtuin1 (Sirt1) but did not ameliorate renal tubular damage and profibrotic genes in the 24 hours and 14 days IRI models. AKI induced NAD+ depletion and impaired autophagy, while augmentation of NAD+ by NR restored tissue NAD+ and increased autophagy, possibly serving as a protective response. However, prophylactic administration of NR did not ameliorate tubular damage of the IRI rats nor rescued the initiation of fibrosis in the long-term AKI to CKD model, which is a pivotal event in CKD pathogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

