Lessons in Organic Fluorescent Probe Discovery
- PMID: 34062039
- PMCID: PMC8595615
- DOI: 10.1002/cbic.202100171
Lessons in Organic Fluorescent Probe Discovery
Abstract
Fluorescent probes have gained profound use in biotechnology, drug discovery, medical diagnostics, molecular and cell biology. The development of methods for the translation of fluorophores into fluorescent probes continues to be a robust field for medicinal chemists and chemical biologists, alike. Access to new experimental designs has enabled molecular diversification and led to the identification of new approaches to probe discovery. This review provides a synopsis of the recent lessons in modern fluorescent probe discovery.
Keywords: chemical biology; fluorescence; fluorophores; molecular probes; sensors.
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures













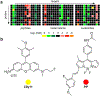



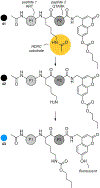
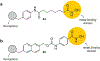







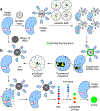






Similar articles
-
Combinatorial discovery of fluorescent pharmacophores by multicomponent reactions in droplet arrays.J Am Chem Soc. 2011 Jul 6;133(26):10058-61. doi: 10.1021/ja204016e. Epub 2011 Jun 14. J Am Chem Soc. 2011. PMID: 21644551
-
Fluorescent probes for real-time measurement of nitric oxide in living cells.Analyst. 2015 Nov 7;140(21):7129-41. doi: 10.1039/c5an01628b. Analyst. 2015. PMID: 26373251 Review.
-
Discovery of novel zebrafish neural tracers by organism-based screening of a rosamine library.Chem Commun (Camb). 2010 May 7;46(17):2932-4. doi: 10.1039/b920432f. Epub 2010 Mar 23. Chem Commun (Camb). 2010. PMID: 20386827
-
Chemical Tools with Fluorescence Switches for Verifying Epigenetic Modifications.Acc Chem Res. 2019 Oct 15;52(10):2849-2857. doi: 10.1021/acs.accounts.9b00349. Epub 2019 Oct 2. Acc Chem Res. 2019. PMID: 31577127 Review.
-
Organic fluorescent probes for monitoring autophagy in living cells.Chem Soc Rev. 2021 Jan 7;50(1):102-119. doi: 10.1039/d0cs00896f. Epub 2020 Nov 6. Chem Soc Rev. 2021. PMID: 33155002 Review.
Cited by
-
Thermal Stability of Fluorescent Chitosan Modified with Heterocyclic Aromatic Dyes.Materials (Basel). 2022 May 20;15(10):3667. doi: 10.3390/ma15103667. Materials (Basel). 2022. PMID: 35629691 Free PMC article.
-
Synthesis of Pyridin-1(2H)-ylacrylates and the Effects of Different Functional Groups on Their Fluorescence.Molecules. 2023 Sep 8;28(18):6511. doi: 10.3390/molecules28186511. Molecules. 2023. PMID: 37764287 Free PMC article.
-
Three-component assembly of stabilized fluorescent isoindoles.RSC Adv. 2022 Mar 2;12(11):6947-6950. doi: 10.1039/d2ra00505k. eCollection 2022 Feb 22. RSC Adv. 2022. PMID: 35424591 Free PMC article.
-
Medicinal chemical optimization of fluorescent pyrrole-based COX-2 probes.Tetrahedron. 2022 Sep 24;123:132990. doi: 10.1016/j.tet.2022.132990. Epub 2022 Aug 28. Tetrahedron. 2022. PMID: 36968982 Free PMC article.
-
Differentiating carrier protein interactions in biosynthetic pathways using dapoxyl solvatochromism.Chem Sci. 2024 Oct 30;15(47):19913-19919. doi: 10.1039/d4sc05499g. eCollection 2024 Dec 4. Chem Sci. 2024. PMID: 39568935 Free PMC article.
References
-
- Monardes N in Historia medicinal de las cosas que se traen de nuestras Indias Occidentales, Seville, 1565.
-
- Sahagún B in 1499–1590, Florentine Codex: General History of the Things of New Spain, 2nd ed., Revised, Santa Fe, N. M.: The School Of American Research; Salt Lake City, Utah, University Of Utah, 1970.
-
- Acuña AU, Amat-Guerri F, Morcillo P, Liras M, Rodriguez B, Org. Lett 2009, 11, 3020–3023; - PubMed
- Tomalia DA, Klajnert-Maculewicz B, Johnson KA-M, Brinkman HF, Janaszewska A, Hedstrand DM, Prog. Polym. Sci 2019, 90, 35–117.
-
- Acuña AU, Amat-Guerri F, in Fluorescence of Supermolecules, Polymers, and Nanosystems, Springer, 2007, pp. 3–20.
-
- Specht EA, Braselmann E, Palmer AE, Annu. Rev. Physiol 2017, 79, 93–117. - PubMed

