Angelica gigas root ameliorates ischaemic stroke-induced brain injury in mice by activating the PI3K/AKT/mTOR and MAPK pathways
- PMID: 34062098
- PMCID: PMC8172223
- DOI: 10.1080/13880209.2021.1928241
Angelica gigas root ameliorates ischaemic stroke-induced brain injury in mice by activating the PI3K/AKT/mTOR and MAPK pathways
Abstract
Context: Traditionally, the root of Angelica gigas Nakai (Umbelliferae), has long been used to treat ischaemic diseases and is considered safe in humans.
Objective: To investigate the neuroprotective effects of a methanol extract of A. gigas root (AGmex) on the middle cerebral artery occlusion (MCAO)-induced brain injury in mice, and the underlying mechanisms.
Materials and methods: Two hours of transient MCAO (tMCAO) was induced in C57BL/6 mice (MCAO control group and AGmex groups), AGmex was administered to the AGmex group at 300-3,000 mg/kg bw at 1, 1, and 24 h before tMCAO or at 1000 mg/kg bw at 1 h before and after tMCAO. Infarction volumes, tissue staining, and western blotting were used to investigate the mechanism underlying the neuroprotective effects of AGmex.
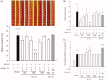
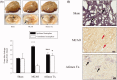
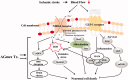
Results: The median effective dose (ED50) could not be measured because the AGmex treatment did not reduce the infarction volume caused by 2 h of tMCAO to within 50%; however, pre-treatment with AGmex twice at 1,000 mg/kg bw before tMCAO significantly reduced the infarction volumes. The proteins related to cell growth, differentiation, and death were upregulated by this treatment, and the major recovery mechanisms appeared to involve the attenuation of the mitochondrial function of Bcl-2/Bax and activation of the PI3K/AKT/mTOR and MAPK signalling pathways in ischaemic neurons.
Conclusions: This study provides evidence supporting the use of A. gigas root against ischaemic stroke and suggests a novel developmental starting point for the treatment of ischaemic stroke.
Keywords: Middle cerebral artery occlusion; brain edoema; infarction; neuroprotection.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





References
-
- Ambe K, Watanabe H, Takahashi S, Nakagawa T.. 2014. Immunohistochemical localization of Nox1, Nox4 and Mn-SOD in mouse femur during endochondral ossification. Tissue Cell. 46:433–438. - PubMed
-
- Choi HS, Cho SG, Kim MK, Kim MS, Moon SH, Kim IH, Ko SG.. 2016. Decursin in Angelica gigas Nakai (AGN) enhances doxorubicin chemosensitivity in NCI/ADR-RES ovarian cancer cells via inhibition of p-glycoprotein expression. Phytother Res. 30:2020–2026. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
