Similarities and differences in the conformational stability and reversibility of ORF8, an accessory protein of SARS-CoV-2, and its L84S variant
- PMID: 34062392
- PMCID: PMC8149210
- DOI: 10.1016/j.bbrc.2021.05.074
Similarities and differences in the conformational stability and reversibility of ORF8, an accessory protein of SARS-CoV-2, and its L84S variant
Abstract
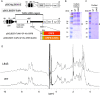
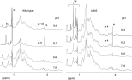
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has the characteristic accessory protein ORF8. Although clinical reports indicate that ORF8 variant strains (Δ382 and L84S variants) are less likely to cause severe illness, functional differences between wild-type and variant ORF8 are unknown. Furthermore, the physicochemical properties of the ORF8 protein have not been analyzed. In this study, the physicochemical properties of the wild-type ORF8 and its L84S variant were analyzed and compared. Using the tobacco BY-2 cell production system, which has been successfully used to produce the wild-type ORF8 protein with a single conformation, was used to successfully produce the ORF8 L84S variant protein at the same level as wild-type ORF8. The produced proteins were purified, and their temperature and pH dependencies were examined using nuclear magnetic resonance spectra. Our data suggested that the wild-type and L84S variant ORF8 structures are highly stable over a wide temperature range. Both proteins displayed an aggregated conformation at higher temperature that reverted when the temperature was decreased to room temperature. Moreover, ORF8 precipitated at acidic pH and this precipitation was reversed when the solution pH was shifted to neutral. Interestingly, the L84S variant exhibited greater solubility than wild-type ORF8 under acidic conditions. Thus, the finding indicated that conformational stability and reversibility of ORF8 are key properties related to function in oppressive environments.
Keywords: L84S variant; ORF8; Protein conformation; Protein stability; Reversibility; SARS-CoV-2.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
SARS-CoV-2 ORF8: One protein, seemingly one structure, and many functions.Front Immunol. 2022 Oct 24;13:1035559. doi: 10.3389/fimmu.2022.1035559. eCollection 2022. Front Immunol. 2022. PMID: 36353628 Free PMC article. Review.
-
Structural and functional effects of the L84S mutant in the SARS-COV-2 ORF8 dimer based on microsecond molecular dynamics study.J Biomol Struct Dyn. 2024 Jul;42(11):5770-5787. doi: 10.1080/07391102.2023.2228919. Epub 2023 Jul 4. J Biomol Struct Dyn. 2024. PMID: 37403295
-
Viral Mimicry of Interleukin-17A by SARS-CoV-2 ORF8.mBio. 2022 Apr 26;13(2):e0040222. doi: 10.1128/mbio.00402-22. Epub 2022 Mar 28. mBio. 2022. PMID: 35343786 Free PMC article.
-
Evolutionary dynamics of the SARS-CoV-2 ORF8 accessory gene.Infect Genet Evol. 2020 Nov;85:104525. doi: 10.1016/j.meegid.2020.104525. Epub 2020 Sep 2. Infect Genet Evol. 2020. PMID: 32890763 Free PMC article.
-
Lost in deletion: The enigmatic ORF8 protein of SARS-CoV-2.Biochem Biophys Res Commun. 2021 Jan 29;538:116-124. doi: 10.1016/j.bbrc.2020.10.045. Epub 2020 Oct 21. Biochem Biophys Res Commun. 2021. PMID: 33685621 Free PMC article. Review.
Cited by
-
Establishing reference sequences for each clade of SARS-CoV-2 to provide a basis for virus variation and function research.J Med Virol. 2022 Apr;94(4):1494-1501. doi: 10.1002/jmv.27476. Epub 2021 Dec 1. J Med Virol. 2022. PMID: 34821382 Free PMC article.
-
Recent development of surface-enhanced Raman scattering for biosensing.J Nanobiotechnology. 2023 May 6;21(1):149. doi: 10.1186/s12951-023-01890-7. J Nanobiotechnology. 2023. PMID: 37149605 Free PMC article. Review.
-
SARS-CoV-2 ORF8: One protein, seemingly one structure, and many functions.Front Immunol. 2022 Oct 24;13:1035559. doi: 10.3389/fimmu.2022.1035559. eCollection 2022. Front Immunol. 2022. PMID: 36353628 Free PMC article. Review.
-
Crystal Structures of Bat and Human Coronavirus ORF8 Protein Ig-Like Domain Provide Insights Into the Diversity of Immune Responses.Front Immunol. 2021 Dec 17;12:807134. doi: 10.3389/fimmu.2021.807134. eCollection 2021. Front Immunol. 2021. PMID: 34975921 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

