CRISPR is a useful biological tool for detecting nucleic acid of SARS-CoV-2 in human clinical samples
- PMID: 34062417
- PMCID: PMC8156908
- DOI: 10.1016/j.biopha.2021.111772
CRISPR is a useful biological tool for detecting nucleic acid of SARS-CoV-2 in human clinical samples
Abstract
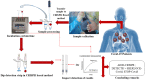
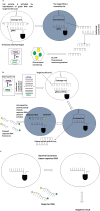
The recent pandemic of novel coronavirus disease (COVID-19) has spread globally and infected millions of people. The quick and specific detection of the nucleic acid of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) remains a challenge within healthcare providers. Currently, quantitative reverse transcription-polymerase chain reaction (RT-qPCR) is the widely used method to detect the SARS-CoV-2 from the human clinical samples. RT-qPCR is expensive equipment and needs skilled personnel as well as lengthy detection time. RT-qPCR limitation needed an alternative healthcare technique to overcome with a fast and cheaper detection method. By applying the principles of CRISPR technology, several promising detection methods giving hope to the healthcare community. CRISPR-based detection methods include SHERLOCK-Covid, STOP-Covid, AIOD-CRISPR, and DETECTR platform. These methods have comparative advantages and drawbacks. Among these methods, AIOD-CRISPR and DETECTR are reasonably better diagnostic methods than the others if we compare the time taken for the test, the cost associated with each test, and their capability of detecting SARS-CoV-2 in the clinical samples. It may expect that the promising CRISPR-based methods would facilitate point-of-care (POC) applications in the CRISPR-built next-generation novel coronavirus diagnostics.
Keywords: COVID-19; CRISPR; Diagnostics; Nucleic acid; SARS-CoV-2.
Copyright © 2021. Published by Elsevier Masson SAS.
Conflict of interest statement
The authors declare no conflict of interest. The authors are solely responsible for the writing and content of this article.
Figures
Similar articles
-
Rapid, Sensitive, and Specific Severe Acute Respiratory Syndrome Coronavirus 2 Detection: A Multicenter Comparison Between Standard Quantitative Reverse-Transcriptase Polymerase Chain Reaction and CRISPR-Based DETECTR.J Infect Dis. 2021 Feb 3;223(2):206-213. doi: 10.1093/infdis/jiaa641. J Infect Dis. 2021. PMID: 33535237 Free PMC article.
-
FnCas9-based CRISPR diagnostic for rapid and accurate detection of major SARS-CoV-2 variants on a paper strip.Elife. 2021 Jun 9;10:e67130. doi: 10.7554/eLife.67130. Elife. 2021. PMID: 34106048 Free PMC article.
-
One-tube SARS-CoV-2 detection platform based on RT-RPA and CRISPR/Cas12a.J Transl Med. 2021 Feb 16;19(1):74. doi: 10.1186/s12967-021-02741-5. J Transl Med. 2021. PMID: 33593370 Free PMC article.
-
CRISPR-based tools: Alternative methods for the diagnosis of COVID-19.Clin Biochem. 2021 Mar;89:1-13. doi: 10.1016/j.clinbiochem.2020.12.011. Epub 2021 Jan 9. Clin Biochem. 2021. PMID: 33428900 Free PMC article. Review.
-
SARS-CoV-2 pandemics: An update of CRISPR in diagnosis and host-virus interaction studies.Biomed J. 2023 Apr;46(2):100587. doi: 10.1016/j.bj.2023.02.007. Epub 2023 Feb 25. Biomed J. 2023. PMID: 36849044 Free PMC article. Review.
Cited by
-
Four thermostatic steps: A novel CRISPR-Cas12-based system for the rapid at-home detection of respiratory pathogens.Appl Microbiol Biotechnol. 2023 Jun;107(12):3983-3996. doi: 10.1007/s00253-023-12568-3. Epub 2023 May 11. Appl Microbiol Biotechnol. 2023. PMID: 37166482 Free PMC article.
-
Rational Programming of Cas12a for Early-Stage Detection of COVID-19 by Lateral Flow Assay and Portable Real-Time Fluorescence Readout Facilities.Biosensors (Basel). 2021 Dec 26;12(1):11. doi: 10.3390/bios12010011. Biosensors (Basel). 2021. PMID: 35049639 Free PMC article.
-
Advancements in SARS-CoV-2 detection: Navigating the molecular landscape and diagnostic technologies.Heliyon. 2024 Apr 21;10(9):e29909. doi: 10.1016/j.heliyon.2024.e29909. eCollection 2024 May 15. Heliyon. 2024. PMID: 38707469 Free PMC article. Review.
-
Accuracy of clustered regularly interspaced short palindromic repeats (CRISPR) to diagnose COVID-19, a meta-analysis.Microb Pathog. 2022 Apr;165:105498. doi: 10.1016/j.micpath.2022.105498. Epub 2022 Mar 25. Microb Pathog. 2022. PMID: 35341958 Free PMC article.
-
Advancing CRISPR-Based Solutions for COVID-19 Diagnosis and Therapeutics.Cells. 2024 Oct 30;13(21):1794. doi: 10.3390/cells13211794. Cells. 2024. PMID: 39513901 Free PMC article. Review.
References
-
- Mahase E., Kmietowicz Z. Covid-19: doctors are told not to perform CPR on patients in cardiac arrest. BMJ. 2020;368:1282. - PubMed
-
- Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J.P., Alvarado-Arnez L.E., Bonilla-Aldana D.K., Franco-Paredes C., Henao-Martinez A.F., Paniz-Mondolfi A., Lagos-Grisales G.J., Ramírez-Vallejo E., Suárez J.A., Zambrano L.I., Villamil-Gómez W.E., Balbin-Ramon G.J., Rabaan A.A., Harapan H., Dhama K., Nishiura H., Kataoka H., Ahmad T., Sah R., Latin American Network of Coronavirus Disease -COVID- Research (LANCOVID-). Electronic address h. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel. Med. Infect. Dis. 2020;34 - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

