Neuroinflammation in Sepsis: Molecular Pathways of Microglia Activation
- PMID: 34062710
- PMCID: PMC8147235
- DOI: 10.3390/ph14050416
Neuroinflammation in Sepsis: Molecular Pathways of Microglia Activation
Abstract
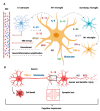
Frequently underestimated, encephalopathy or delirium are common neurological manifestations associated with sepsis. Brain dysfunction occurs in up to 80% of cases and is directly associated with increased mortality and long-term neurocognitive consequences. Although the central nervous system (CNS) has been classically viewed as an immune-privileged system, neuroinflammation is emerging as a central mechanism of brain dysfunction in sepsis. Microglial cells are major players in this setting. Here, we aimed to discuss the current knowledge on how the brain is affected by peripheral immune activation in sepsis and the role of microglia in these processes. This review focused on the molecular pathways of microglial activity in sepsis, its regulatory mechanisms, and their interaction with other CNS cells, especially with neuronal cells and circuits.
Keywords: brain; inflammasome; microglia; neuroinflammation; neurotoxicity; oxidative stress; sepsis-associated encephalopathy; synaptic dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Neuroinflammation: microglial activation during sepsis.Curr Neurovasc Res. 2014;11(3):262-70. doi: 10.2174/1567202611666140520122744. Curr Neurovasc Res. 2014. PMID: 24845857 Review.
-
Microglia: A Potential Therapeutic Target for Sepsis-Associated Encephalopathy and Sepsis-Associated Chronic Pain.Front Pharmacol. 2020 Nov 27;11:600421. doi: 10.3389/fphar.2020.600421. eCollection 2020. Front Pharmacol. 2020. PMID: 33329005 Free PMC article. Review.
-
Central role of microglia in sepsis-associated encephalopathy: From mechanism to therapy.Front Immunol. 2022 Jul 26;13:929316. doi: 10.3389/fimmu.2022.929316. eCollection 2022. Front Immunol. 2022. PMID: 35958583 Free PMC article. Review.
-
Hydrogen Alleviates Neuronal Injury and Neuroinflammation Induced by Microglial Activation via the Nuclear Factor Erythroid 2-related Factor 2 Pathway in Sepsis-associated Encephalopathy.Neuroscience. 2021 Jul 1;466:87-100. doi: 10.1016/j.neuroscience.2021.05.003. Epub 2021 May 13. Neuroscience. 2021. PMID: 33992722
-
Microglial Activation: Key Players in Sepsis-Associated Encephalopathy.Brain Sci. 2023 Oct 12;13(10):1453. doi: 10.3390/brainsci13101453. Brain Sci. 2023. PMID: 37891821 Free PMC article. Review.
Cited by
-
Reactive Microgliosis in Sepsis-Associated and Acute Hepatic Encephalopathies: An Ultrastructural Study.Int J Mol Sci. 2022 Nov 21;23(22):14455. doi: 10.3390/ijms232214455. Int J Mol Sci. 2022. PMID: 36430933 Free PMC article.
-
Neuroinflammation in the medullary visceral zone exert a powerful impaction on the systemic inflammation in sepsis through cholinergic anti-inflammatory pathway.Sci Rep. 2024 Jul 23;14(1):16921. doi: 10.1038/s41598-024-67531-7. Sci Rep. 2024. PMID: 39043772 Free PMC article.
-
Cytokine profiles in intensive care unit delirium.Acute Crit Care. 2022 Aug;37(3):415-428. doi: 10.4266/acc.2021.01508. Epub 2022 Jun 20. Acute Crit Care. 2022. PMID: 35791660 Free PMC article.
-
Autocrine positive feedback of tumor necrosis factor from activated microglia proposed to be of widespread relevance in chronic neurological disease.Pharmacol Res Perspect. 2023 Oct;11(5):e01136. doi: 10.1002/prp2.1136. Pharmacol Res Perspect. 2023. PMID: 37750203 Free PMC article. Review.
-
Electroacupuncture Alleviates Neuroinflammation by Inhibiting the HMGB1 Signaling Pathway in Rats with Sepsis-Associated Encephalopathy.Brain Sci. 2022 Dec 17;12(12):1732. doi: 10.3390/brainsci12121732. Brain Sci. 2022. PMID: 36552192 Free PMC article.
References
-
- Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
-
- Nielsen R.M., Urdanibia-Centelles O., Vedel-Larsen E., Thomsen K.J., Møller K., Olsen K.S., Lauritsen A.Ø., Eddelien H.S., Lauritzen M., Benedek K. Continuous EEG Monitoring in a Consecutive Patient Cohort with Sepsis and Delirium. Neurocrit. Care. 2020;32:121–130. doi: 10.1007/s12028-019-00703-w. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

