Nipagin-Functionalized Porphyrazine and Phthalocyanine-Synthesis, Physicochemical Characterization and Toxicity Study after Deposition on Titanium Dioxide Nanoparticles P25
- PMID: 34062815
- PMCID: PMC8124671
- DOI: 10.3390/molecules26092657
Nipagin-Functionalized Porphyrazine and Phthalocyanine-Synthesis, Physicochemical Characterization and Toxicity Study after Deposition on Titanium Dioxide Nanoparticles P25
Abstract
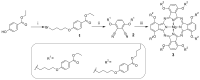
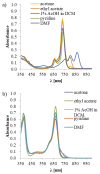
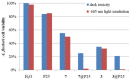
Aza-porphyrinoids exhibit distinct spectral properties in UV-Vis, and they are studied in applications such as photosensitizers in medicine and catalysts in technology. The use of appropriate peripheral substituents allows the modulation of their physicochemical properties. Phthalocyanine and sulfanyl porphyrazine octa-substituted with 4-(butoxycarbonyl)phenyloxy moieties were synthesized and characterized using UV-Vis and NMR spectroscopy, as well as mass spectrometry. A comparison of porphyrazine with phthalocyanine aza-porphyrinoids revealed that phthalocyanine macrocycle exhibits higher singlet oxygen generation quantum yields, reaching the value of 0.29 in DMF. After both macrocycles had been deposited on titanium dioxide nanoparticles P25, the cytotoxicities and photocytotoxicities of the prepared materials were studied using a Microtox® acute toxicity test. The highest cytotoxicity occurred after irradiation with a red light for the material composed of phthalocyanine deposited on titania nanoparticles.
Keywords: Linstead macrocyclization; Microtox; phthalocyanine; porphyrazine; singlet oxygen; titanium dioxide.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Rodríguez-Morgade M.S., Stuzhin P.A. The chemistry of porphyrazines: An overview. J. Porphyrins Phthalocyanines. 2004;8:1129–1165. doi: 10.1142/S1088424604000490. - DOI
-
- Falkowski M., Rebis T., Piskorz J., Popenda L., Jurga S., Mielcarek J., Milczarek G., Goslinski T. Multiwalled carbon nanotube/sulfanyl porphyrazine hybrids deposited on glassy carbon electrode—Effect of nitro peripheral groups on electrochemical properties. J. Porphyrins Phthalocyanines. 2017;21:295–301. doi: 10.1142/S1088424617500134. - DOI
-
- Olgaç R., Baygu Y., Yıldız B., Gök Y., Köksoy B., Durmuş M. Synthesis, characterization and photochemical properties of metallo porphyrazines substituted with alkyl linked carbazole, benzoazepine and phenothiazine moieties. J. Porphyrins Phthalocyanines. 2017;21:599–610. doi: 10.1142/S1088424617500596. - DOI
-
- Chełminiak-Dudkiewicz D., Ziegler-Borowska M., Stolarska M., Sobotta L., Falkowski M., Mielcarek J., Goslinski T., Kowalonek J., Węgrzynowska-Drzymalska K., Kaczmarek H. The chitosan—Porphyrazine hybrid materials and their photochemical properties. J. Photochemistry Photobiol. B Biol. 2018;181:1–13. doi: 10.1016/j.jphotobiol.2018.02.021. - DOI - PubMed
-
- Yang C., Gao L., Zhang B., Zhang Z., Deng K. Uniform Zinc Thioporphyrazine nanosphere by self-assembly and the photocatalytic performance. J. Porphyrins Phthalocyanines. 2018;22:868–876. doi: 10.1142/S1088424618500487. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

