Natural Killer-Dendritic Cell Interactions in Liver Cancer: Implications for Immunotherapy
- PMID: 34062821
- PMCID: PMC8124166
- DOI: 10.3390/cancers13092184
Natural Killer-Dendritic Cell Interactions in Liver Cancer: Implications for Immunotherapy
Abstract
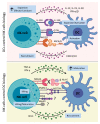
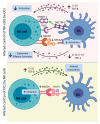
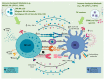
Natural killer (NK) and dendritic cells (DCs) are innate immune cells that play a crucial role in anti-tumor immunity. NK cells kill tumor cells through direct cytotoxicity and cytokine secretion. DCs are needed for the activation of adaptive immune responses against tumor cells. Both NK cells and DCs are subdivided in several subsets endowed with specialized effector functions. Crosstalk between NK cells and DCs leads to the reciprocal control of their activation and polarization of immune responses. In this review, we describe the role of NK cells and DCs in liver cancer, focusing on the mechanisms involved in their reciprocal control and activation. In this context, intrahepatic NK cells and DCs present unique immunological features, due to the constant exposure to non-self-circulating antigens. These interactions might play a fundamental role in the pathology of primary liver cancer, namely hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC). Additionally, the implications of these immune changes are relevant from the perspective of improving the cancer immunotherapy strategies in HCC and ICC patients.
Keywords: DCs–NK cell interaction; cancer therapy; innate immunity; liver cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures