AGMO Inhibitor Reduces 3T3-L1 Adipogenesis
- PMID: 34062826
- PMCID: PMC8147360
- DOI: 10.3390/cells10051081
AGMO Inhibitor Reduces 3T3-L1 Adipogenesis
Abstract
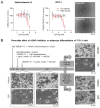
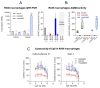
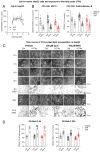
Alkylglycerol monooxygenase (AGMO) is a tetrahydrobiopterin (BH4)-dependent enzyme with major expression in the liver and white adipose tissue that cleaves alkyl ether glycerolipids. The present study describes the disclosure and biological characterization of a candidate compound (Cp6), which inhibits AGMO with an IC50 of 30-100 µM and 5-20-fold preference of AGMO relative to other BH4-dependent enzymes, i.e., phenylalanine-hydroxylase and nitric oxide synthase. The viability and metabolic activity of mouse 3T3-L1 fibroblasts, HepG2 human hepatocytes and mouse RAW264.7 macrophages were not affected up to 10-fold of the IC50. However, Cp6 reversibly inhibited the differentiation of 3T3-L1 cells towards adipocytes, in which AGMO expression was upregulated upon differentiation. Cp6 reduced the accumulation of lipid droplets in adipocytes upon differentiation and in HepG2 cells exposed to free fatty acids. Cp6 also inhibited IL-4-driven differentiation of RAW264.7 macrophages towards M2-like macrophages, which serve as adipocyte progenitors in adipose tissue. Collectively, the data suggest that pharmacologic AGMO inhibition may affect lipid storage.
Keywords: 3T3-L1 mouse fibroblasts; AGMO; adipocytes; compound screen; enzyme activity assay; macrophage polarization; tetrahydrobiopterin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






Similar articles
-
Adaptations of the 3T3-L1 adipocyte lipidome to defective ether lipid catabolism upon Agmo knockdown.J Lipid Res. 2022 Jun;63(6):100222. doi: 10.1016/j.jlr.2022.100222. Epub 2022 May 7. J Lipid Res. 2022. PMID: 35537527 Free PMC article.
-
Fenretinide mediated retinoic acid receptor signalling and inhibition of ceramide biosynthesis regulates adipogenesis, lipid accumulation, mitochondrial function and nutrient stress signalling in adipocytes and adipose tissue.Biochem Pharmacol. 2016 Jan 15;100:86-97. doi: 10.1016/j.bcp.2015.11.017. Epub 2015 Nov 22. Biochem Pharmacol. 2016. PMID: 26592777 Free PMC article.
-
In vitro anti-diabetic effect of flavonoids and pheophytins from Allophylus cominia Sw. on the glucose uptake assays by HepG2, L6, 3T3-L1 and fat accumulation in 3T3-L1 adipocytes.J Ethnopharmacol. 2018 Apr 24;216:8-17. doi: 10.1016/j.jep.2018.01.014. Epub 2018 Jan 12. J Ethnopharmacol. 2018. PMID: 29339110
-
Glycine enhances expression of adiponectin and IL-10 in 3T3-L1 adipocytes without affecting adipogenesis and lipolysis.Amino Acids. 2018 May;50(5):629-640. doi: 10.1007/s00726-018-2537-3. Epub 2018 Jan 22. Amino Acids. 2018. PMID: 29356901
-
The Emerging Physiological Role of AGMO 10 Years after Its Gene Identification.Life (Basel). 2021 Jan 26;11(2):88. doi: 10.3390/life11020088. Life (Basel). 2021. PMID: 33530536 Free PMC article. Review.
Cited by
-
Transcriptome in Liver of Periparturient Dairy Cows Differs between Supplementation of Rumen-Protected Niacin and Rumen-Protected Nicotinamide.Metabolites. 2024 Mar 1;14(3):150. doi: 10.3390/metabo14030150. Metabolites. 2024. PMID: 38535310 Free PMC article.
-
Adaptations of the 3T3-L1 adipocyte lipidome to defective ether lipid catabolism upon Agmo knockdown.J Lipid Res. 2022 Jun;63(6):100222. doi: 10.1016/j.jlr.2022.100222. Epub 2022 May 7. J Lipid Res. 2022. PMID: 35537527 Free PMC article.
-
Genomic Analyses for Selective Signatures and Genes Involved in Hot Adaptation Among Indigenous Chickens From Different Tropical Climate Regions.Front Genet. 2022 Jul 22;13:906447. doi: 10.3389/fgene.2022.906447. eCollection 2022. Front Genet. 2022. PMID: 35979430 Free PMC article.
-
The Association between CDKAL1 Gene rs10946398 Polymorphism and Post-Transplant Diabetes in Kidney Allograft Recipients Treated with Tacrolimus.Genes (Basel). 2023 Aug 7;14(8):1595. doi: 10.3390/genes14081595. Genes (Basel). 2023. PMID: 37628646 Free PMC article.
References
-
- Pike L.J., Han X., Chung K.N., Gross R.W. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: A quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–2088. doi: 10.1021/bi0156557. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

