New Hybrids Based on Curcumin and Resveratrol: Synthesis, Cytotoxicity and Antiproliferative Activity against Colorectal Cancer Cells
- PMID: 34062841
- PMCID: PMC8124228
- DOI: 10.3390/molecules26092661
New Hybrids Based on Curcumin and Resveratrol: Synthesis, Cytotoxicity and Antiproliferative Activity against Colorectal Cancer Cells
Abstract
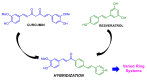
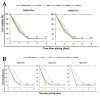
We synthesized twelve hybrids based on curcumin and resveratrol, and their structures were elucidated by spectroscopic analysis. The chemopreventive potential of these compounds was evaluated against SW480 human colon adenocarcinoma cells, its metastatic derivative SW620, along with the non-malignant CHO-K1 cell line. Among the tested compounds, hybrids 3e and 3i (for SW480) and 3a, 3e and 3k (for SW620) displayed the best cytotoxic activity with IC50 values ranging from 11.52 ± 2.78 to 29.33 ± 4.73 µM for both cell lines, with selectivity indices (SI) higher than 1, after 48 h of treatment. Selectivity indices were even higher than those reported for the reference drug, 5-fluorouracil (SI = 0.96), the starting compound resveratrol (SI = 0.45) and the equimolar mixture of curcumin plus resveratrol (SI = 0.77). The previous hybrids showed good antiproliferative activity.
Keywords: antiproliferative activity; colorectal cancer; curcumin; cytotoxicity; hybrid molecules; resveratrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
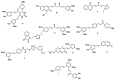
References
-
- Aggarwal B.B., Kumar A., Bharti A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer. Res. 2003;23:363–398. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

