N-Glycolylneuraminic Acid in Animal Models for Human Influenza A Virus
- PMID: 34062844
- PMCID: PMC8147317
- DOI: 10.3390/v13050815
N-Glycolylneuraminic Acid in Animal Models for Human Influenza A Virus
Abstract
The first step in influenza virus infection is the binding of hemagglutinin to sialic acid-containing glycans present on the cell surface. Over 50 different sialic acid modifications are known, of which N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc) are the two main species. Animal models with α2,6 linked Neu5Ac in the upper respiratory tract, similar to humans, are preferred to enable and mimic infection with unadapted human influenza A viruses. Animal models that are currently most often used to study human influenza are mice and ferrets. Additionally, guinea pigs, cotton rats, Syrian hamsters, tree shrews, domestic swine, and non-human primates (macaques and marmosets) are discussed. The presence of NeuGc and the distribution of sialic acid linkages in the most commonly used models is summarized and experimentally determined. We also evaluated the role of Neu5Gc in infection using Neu5Gc binding viruses and cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH)-/- knockout mice, which lack Neu5Gc and concluded that Neu5Gc is unlikely to be a decoy receptor. This article provides a base for choosing an appropriate animal model. Although mice are one of the most favored models, they are hardly naturally susceptible to infection with human influenza viruses, possibly because they express mainly α2,3 linked sialic acids with both Neu5Ac and Neu5Gc modifications. We suggest using ferrets, which resemble humans closely in the sialic acid content, both in the linkages and the lack of Neu5Gc, lung organization, susceptibility, and disease pathogenesis.
Keywords: CMAH; N-acetylneuraminic acid; N-glycolylneuraminic acid; animal model; ferret; influenza; mouse; sialic acid linkage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

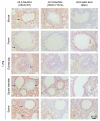

Similar articles
-
Ferrets exclusively synthesize Neu5Ac and express naturally humanized influenza A virus receptors.Nat Commun. 2014 Dec 17;5:5750. doi: 10.1038/ncomms6750. Nat Commun. 2014. PMID: 25517696 Free PMC article.
-
Single Particle Analysis of H3N2 Influenza Entry Differentiates the Impact of the Sialic Acids (Neu5Ac and Neu5Gc) on Virus Binding and Membrane Fusion.J Virol. 2023 Mar 30;97(3):e0146322. doi: 10.1128/jvi.01463-22. Epub 2023 Feb 13. J Virol. 2023. PMID: 36779754 Free PMC article.
-
N-glycolylneuraminic acid on human epithelial cells prevents entry of influenza A viruses that possess N-glycolylneuraminic acid binding ability.J Virol. 2014 Aug;88(15):8445-56. doi: 10.1128/JVI.00716-14. Epub 2014 May 14. J Virol. 2014. PMID: 24829344 Free PMC article.
-
[Functional analysis of glyco-molecules that bind with influenza virus].Uirusu. 2016;66(1):101-116. doi: 10.2222/jsv.66.101. Uirusu. 2016. PMID: 28484173 Review. Japanese.
-
Influenza Neuraminidase: Underrated Role in Receptor Binding.Trends Microbiol. 2019 Jun;27(6):477-479. doi: 10.1016/j.tim.2019.03.001. Epub 2019 Mar 29. Trends Microbiol. 2019. PMID: 30930001 Free PMC article. Review.
Cited by
-
Contemporary human H3N2 influenza A viruses require a low threshold of suitable glycan receptors for efficient infection.Glycobiology. 2023 Oct 30;33(10):784-800. doi: 10.1093/glycob/cwad060. Glycobiology. 2023. PMID: 37471650 Free PMC article.
-
Sialic Acid Receptor Specificity in Mammary Gland of Dairy Cattle Infected with Highly Pathogenic Avian Influenza A(H5N1) Virus.Emerg Infect Dis. 2024 Jul;30(7):1361-1373. doi: 10.3201/eid3007.240689. Epub 2024 Jun 11. Emerg Infect Dis. 2024. PMID: 38861554 Free PMC article.
-
An In Silico Functional Analysis of Non-Synonymous Single-Nucleotide Polymorphisms of Bovine CMAH Gene and Potential Implication in Pathogenesis.Pathogens. 2023 Apr 13;12(4):591. doi: 10.3390/pathogens12040591. Pathogens. 2023. PMID: 37111477 Free PMC article.
-
Neuraminidase-mediated enhancement of Streptococcus pneumoniae colonization is associated with altered mucus characteristics and distribution.mBio. 2025 Jan 8;16(1):e0257924. doi: 10.1128/mbio.02579-24. Epub 2024 Dec 11. mBio. 2025. PMID: 39660923 Free PMC article.
-
Mucosal multivalent NDV-based vaccine provides cross-reactive immune responses against SARS-CoV-2 variants in animal models.Front Immunol. 2025 Mar 17;16:1524477. doi: 10.3389/fimmu.2025.1524477. eCollection 2025. Front Immunol. 2025. PMID: 40165947 Free PMC article.
References
-
- Gambaryan A.S., Matrosovich T.Y., Philipp J., Munster V.J., Fouchier R.A., Cattoli G., Capua I., Krauss S.L., Webster R.G., Banks J., et al. Receptor-binding profiles of H7 subtype influenza viruses in different host species. J. Virol. 2012;86:4370–4379. doi: 10.1128/JVI.06959-11. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

