Choosing New Therapies for Gonorrhoea: We Need to Consider the Impact on the Pan- Neisseria Genome. A Viewpoint
- PMID: 34062856
- PMCID: PMC8147325
- DOI: 10.3390/antibiotics10050515
Choosing New Therapies for Gonorrhoea: We Need to Consider the Impact on the Pan- Neisseria Genome. A Viewpoint
Abstract
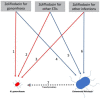
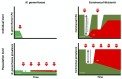
The development of new gonorrhoea treatment guidelines typically considers the resistance-inducing effect of the treatment only on Neisseria gonorrhoeae. Antimicrobial resistance in N. gonorrhoeae has, however, frequently first emerged in commensal Neisseria species and then been passed on to N. gonorrhoeae via transformation. This creates the rationale for considering the effect of gonococcal therapies on resistance in commensal Neisseria. We illustrate the benefits of this pan-Neisseria strategy by evaluating three contemporary treatment options for N. gonorrhoeae-ceftriaxone plus azithromycin, monotherapy with ceftriaxone and zoliflodacin.
Keywords: N. gonorrrhoeae; Neisseria; antimicrobial consumption; antimicrobial resistance; dual therapy.
Conflict of interest statement
The authors state that they have no conflict of interest.
Figures


Similar articles
-
Gonococcal resistance to zoliflodacin could emerge via transformation from commensal Neisseria species. An in-vitro transformation study.Sci Rep. 2024 Jan 12;14(1):1179. doi: 10.1038/s41598-023-49943-z. Sci Rep. 2024. PMID: 38216602 Free PMC article.
-
Current and future antimicrobial treatment of gonorrhoea - the rapidly evolving Neisseria gonorrhoeae continues to challenge.BMC Infect Dis. 2015 Aug 21;15:364. doi: 10.1186/s12879-015-1029-2. BMC Infect Dis. 2015. PMID: 26293005 Free PMC article. Review.
-
Pharmacodynamics of zoliflodacin plus doxycycline combination therapy against Neisseria gonorrhoeae in a gonococcal hollow-fiber infection model.Front Pharmacol. 2023 Dec 7;14:1291885. doi: 10.3389/fphar.2023.1291885. eCollection 2023. Front Pharmacol. 2023. PMID: 38130409 Free PMC article.
-
Antimicrobial susceptibility of Neisseria gonorrhoeae isolates and treatment of gonorrhoea patients in Ternopil and Dnipropetrovsk regions of Ukraine, 2013-2018.APMIS. 2019 Jul;127(7):503-509. doi: 10.1111/apm.12948. Epub 2019 May 21. APMIS. 2019. PMID: 30903707
-
Antimicrobial-resistant gonorrhoea: the national public health response, England, 2013 to 2020.Euro Surveill. 2022 Oct;27(40):2200057. doi: 10.2807/1560-7917.ES.2022.27.40.2200057. Euro Surveill. 2022. PMID: 36205171 Free PMC article. Review.
Cited by
-
Canary in the Coal Mine: How Resistance Surveillance in Commensals Could Help Curb the Spread of AMR in Pathogenic Neisseria.mBio. 2022 Oct 26;13(5):e0199122. doi: 10.1128/mbio.01991-22. Epub 2022 Sep 26. mBio. 2022. PMID: 36154280 Free PMC article. Review.
-
Evidence of horizontal gene transfer within porB in 19 018 whole-genome Neisseria spp. isolates: a global phylogenetic analysis.Microb Genom. 2023 Jun;9(6):mgen001041. doi: 10.1099/mgen.0.001041. Microb Genom. 2023. PMID: 37294009 Free PMC article.
-
Switching From Dual to Monotherapy for Gonorrhea is Associated With a Halving of Gonococcal Resistance to Azithromycin-A Modelling Study of MSM in Belgium.Open Forum Infect Dis. 2025 Jun 2;12(6):ofaf320. doi: 10.1093/ofid/ofaf320. eCollection 2025 Jun. Open Forum Infect Dis. 2025. PMID: 40568000 Free PMC article.
-
Antimicrobial susceptibilities of oral Neisseria from men on HIV pre-exposure prophylaxis in Hanoi, Vietnam.J Glob Antimicrob Resist. 2025 Jan;40:11-14. doi: 10.1016/j.jgar.2024.11.008. Epub 2024 Nov 28. J Glob Antimicrob Resist. 2025. PMID: 39612986 Free PMC article.
-
Variability of antimicrobial susceptibility of commensal Neisseria species supports its use as a marker of excessive antimicrobial consumption - reflections from the results of a four-country study.Int J Infect Dis. 2025 May;154:107870. doi: 10.1016/j.ijid.2025.107870. Epub 2025 Mar 7. Int J Infect Dis. 2025. PMID: 40057265 Free PMC article.
References
-
- Harris S.R., Cole M.J., Spiteri G., Sánchez-Busó L., Golparian D., Jacobsson S., Goater R., Abudahab K., Yeats C.A., Bercot B., et al. Public health surveillance of multidrug-resistant clones of Neisseria gonorrhoeae in Europe: A genomic survey. Lancet Infect. Dis. 2018;18:758–768. doi: 10.1016/S1473-3099(18)30225-1. - DOI - PMC - PubMed
-
- Yin Y.-P., Han Y., Dai X.-Q., Zheng H.-P., Chen S.-C., Zhu B.-Y., Yong G., Zhong N., Hu L.-H., Cao W.-L., et al. Susceptibility of Neisseria gonorrhoeae to azithromycin and ceftriaxone in China: A retrospective study of national surveillance data from 2013 to 2016. PLoS Med. 2018;15:e1002499. doi: 10.1371/journal.pmed.1002499. - DOI - PMC - PubMed
-
- Wi T., Lahra M.M., Ndowa F., Bala M., Dillon J.-A.R., Ramon-Pardo P., Eremin S.R., Bolan G., Unemo M. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017;14:e1002344. doi: 10.1371/journal.pmed.1002344. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

