Zerumbone Inhibits Helicobacter pylori Urease Activity
- PMID: 34062878
- PMCID: PMC8124612
- DOI: 10.3390/molecules26092663
Zerumbone Inhibits Helicobacter pylori Urease Activity
Abstract
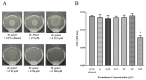
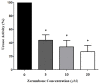
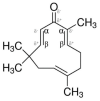
Helicobacter pylori (H. pylori) produces urease in order to improve its settlement and growth in the human gastric epithelium. Urease inhibitors likely represent potentially powerful therapeutics for treating H. pylori; however, their instability and toxicity have proven problematic in human clinical trials. In this study, we investigate the ability of a natural compound extracted from Zingiber zerumbet Smith, zerumbone, to inhibit the urease activity of H. pylori by formation of urease dimers, trimers, or tetramers. As an oxygen atom possesses stronger electronegativity than the first carbon atom bonded to it, in the zerumbone structure, the neighboring second carbon atom shows a relatively negative charge (δ-) and the next carbon atom shows a positive charge (δ+), sequentially. Due to this electrical gradient, it is possible that H. pylori urease with its negative charges (such as thiol radicals) might bind to the β-position carbon of zerumbone. Our results show that zerumbone dimerized, trimerized, or tetramerized with both H. pylori urease A and urease B molecules, and that this formation of complex inhibited H. pylori urease activity. Although zerumbone did not affect either gene transcription or the protein expression of urease A and urease B, our study demonstrated that zerumbone could effectively dimerize with both urease molecules and caused significant functional inhibition of urease activity. In short, our findings suggest that zerumbone may be an effective H. pylori urease inhibitor that may be suitable for therapeutic use in humans.
Keywords: H. pylori; antimicrobial; dimerization; urease; zerumbone.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Antisecretory, gastroprotective, antioxidant and anti-Helicobcter pylori activity of zerumbone from Zingiber zerumbet (L.) Smith.PLoS One. 2015 Mar 23;10(3):e0121060. doi: 10.1371/journal.pone.0121060. eCollection 2015. PLoS One. 2015. Retraction in: PLoS One. 2023 Nov 10;18(11):e0294009. doi: 10.1371/journal.pone.0294009. PMID: 25798602 Free PMC article. Retracted.
-
Selective antibacterial activity of patchouli alcohol against Helicobacter pylori based on inhibition of urease.Phytother Res. 2015 Jan;29(1):67-72. doi: 10.1002/ptr.5227. Epub 2014 Sep 22. Phytother Res. 2015. PMID: 25243578
-
Effect of propolis in gastric disorders: inhibition studies on the growth of Helicobacter pylori and production of its urease.J Enzyme Inhib Med Chem. 2016;31(sup2):46-50. doi: 10.1080/14756366.2016.1186023. Epub 2016 May 27. J Enzyme Inhib Med Chem. 2016. PMID: 27233102
-
Anti-Helicobacter pylori activities of ebrotidine. A review of biochemical and animal experimental studies and data.Arzneimittelforschung. 1997 Apr;47(4A):475-82. Arzneimittelforschung. 1997. PMID: 9205747 Review.
-
Transition Metal/Lewis Acid Catalyzed Reactions of Zerumbone for Diverse Molecular Motifs.Chem Rec. 2021 Dec;21(12):3943-3953. doi: 10.1002/tcr.202100206. Epub 2021 Oct 27. Chem Rec. 2021. PMID: 34708494 Review.
Cited by
-
Towards Effective Helicobacter pylori Eradication: Emerging Therapies in the Wake of Antibiotic Resistance.Int J Mol Sci. 2025 Jun 24;26(13):6064. doi: 10.3390/ijms26136064. Int J Mol Sci. 2025. PMID: 40649842 Free PMC article. Review.
-
Asclepain cI, a proteolytic enzyme from Asclepias curassavica L., a south American plant, against Helicobacter pylori.Front Microbiol. 2022 Aug 18;13:961958. doi: 10.3389/fmicb.2022.961958. eCollection 2022. Front Microbiol. 2022. PMID: 36060760 Free PMC article.
-
New Directions in Helicobacter pylori Urease Inhibitors: Focusing on Nickel Ions Transfer and Auxiliary Protein Interactions During Urease Maturation.Infect Drug Resist. 2025 Jun 17;18:3037-3053. doi: 10.2147/IDR.S519194. eCollection 2025. Infect Drug Resist. 2025. PMID: 40548165 Free PMC article. Review.
-
Healthy Zerumbone: From Natural Sources to Strategies to Improve Its Bioavailability and Oral Administration.Plants (Basel). 2022 Dec 20;12(1):5. doi: 10.3390/plants12010005. Plants (Basel). 2022. PMID: 36616138 Free PMC article. Review.
-
Helicobacter pylori infection in humans and phytotherapy, probiotics, and emerging therapeutic interventions: a review.Front Microbiol. 2024 Jan 10;14:1330029. doi: 10.3389/fmicb.2023.1330029. eCollection 2023. Front Microbiol. 2024. PMID: 38268702 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

