Native α-Synuclein, 3-Nitrotyrosine Proteins, and Patterns of Nitro-α-Synuclein-Immunoreactive Inclusions in Saliva and Submandibulary Gland in Parkinson's Disease
- PMID: 34062880
- PMCID: PMC8147273
- DOI: 10.3390/antiox10050715
Native α-Synuclein, 3-Nitrotyrosine Proteins, and Patterns of Nitro-α-Synuclein-Immunoreactive Inclusions in Saliva and Submandibulary Gland in Parkinson's Disease
Abstract
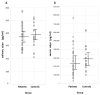
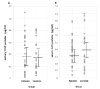
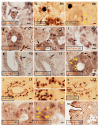
Background. Salivary α-synuclein (aSyn) and its nitrated form, or 3-nitrotyrosine-α-synuclein (3-NT-αSyn), hold promise as biomarkers for idiopathic Parkinson's disease (IPD). Nitrative stress that is characterized by an excess of 3-nitrotyrosine proteins (3-NT-proteins) has been proposed as a pathogenic mechanism in IPD. The objective is to study the pathological role of native αSyn, 3-NT-αSyn, and 3-NT-proteins in the saliva and submandibulary glands of patients with IPD. Methods. The salivary and serum αSyn and 3-NT-proteins concentration is evaluated with ELISA in patients and controls. Correlations of αSyn and 3-NT-proteins content with clinical features of the disease are examined. Immunohistochemical 3-NT-αSyn expression in submandibulary gland sections is analyzed. Results. (a) Salivary concentration and saliva/serum ratios of native αSyn and 3-NT-proteins are similar in patients and controls; (b) salivary αSyn and 3-NT-proteins do not correlate with any clinical feature; and (c) three patterns of 3-NT-αSyn-positive inclusions are observed on histological sections: rounded "Lewy-type" aggregates of 10-25 µm in diameter, coarse deposits with varied morphology, and spheroid inclusions or bodies of 3-5 µm in diameter. "Lewy-type" and coarse inclusions are observed in the interlobular connective tissue of the gland, and small-sized bodies are located within the cytoplasm of duct cells. "Lewy-type" inclusions are only observed in patients, and the remaining patterns of inclusions are observed in both the patients and controls. Conclusions. The patients' saliva presents a similar concentration of native αSyn and 3-nitrotyrosine-proteins than that of the controls, and no correlations with clinical features are found. These findings preclude the utility of native αSyn in the saliva as a biomarker, and they indicate the absence of nitrative stress in the saliva and serum of patients. As regards nitrated αSyn, "Lewy-type" inclusions expressing 3-NT-αSyn are observed in the patients, not the controls-a novel finding that suggests that a biopsy of the submandibulary gland, if proven safe, could be a useful technique for diagnosing IPD. Finally, to our knowledge, this is also the first description of 3-NT-αSyn-immunoreactive intracytoplasmic bodies in cells that are located outside the nervous system. These intracytoplasmic bodies are present in duct cells of submandibulary gland sections from all subjects regardless of their pathology, and they can represent an aging or involutional change. Further immunostaining studies with different antibodies and larger samples are needed to validate the data.
Keywords: lewy-type; nitration; nitrative stress; parkinson; saliva; α-synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
C-Terminal Tyrosine Residue Modifications Modulate the Protective Phosphorylation of Serine 129 of α-Synuclein in a Yeast Model of Parkinson's Disease.PLoS Genet. 2016 Jun 24;12(6):e1006098. doi: 10.1371/journal.pgen.1006098. eCollection 2016 Jun. PLoS Genet. 2016. PMID: 27341336 Free PMC article.
-
Human myeloperoxidase (hMPO) is expressed in neurons in the substantia nigra in Parkinson's disease and in the hMPO-α-synuclein-A53T mouse model, correlating with increased nitration and aggregation of α-synuclein and exacerbation of motor impairment.Free Radic Biol Med. 2019 Sep;141:115-140. doi: 10.1016/j.freeradbiomed.2019.05.033. Epub 2019 Jun 6. Free Radic Biol Med. 2019. PMID: 31175983 Free PMC article.
-
Salivary ATP13A2 is a potential marker of therapy-induced motor complications and is expressed by inclusions in submandibulary glands in Parkinson ´s disease.Clin Park Relat Disord. 2022 Aug 28;7:100163. doi: 10.1016/j.prdoa.2022.100163. eCollection 2022. Clin Park Relat Disord. 2022. PMID: 36081833 Free PMC article.
-
Gangliosides, α-Synuclein, and Parkinson's Disease.Prog Mol Biol Transl Sci. 2018;156:435-454. doi: 10.1016/bs.pmbts.2017.12.009. Epub 2018 Feb 24. Prog Mol Biol Transl Sci. 2018. PMID: 29747823 Review.
-
Quantification of α-synuclein in cerebrospinal fluid: how ideal is this biomarker for Parkinson's disease?Parkinsonism Relat Disord. 2014 Jan;20 Suppl 1:S76-9. doi: 10.1016/S1353-8020(13)70020-8. Parkinsonism Relat Disord. 2014. PMID: 24262194 Review.
Cited by
-
Protein Oxidative Modifications in Neurodegenerative Diseases: From Advances in Detection and Modelling to Their Use as Disease Biomarkers.Antioxidants (Basel). 2024 May 31;13(6):681. doi: 10.3390/antiox13060681. Antioxidants (Basel). 2024. PMID: 38929122 Free PMC article. Review.
-
Changes in α-Synuclein Posttranslational Modifications in an AAV-Based Mouse Model of Parkinson's Disease.Int J Mol Sci. 2023 Aug 30;24(17):13435. doi: 10.3390/ijms241713435. Int J Mol Sci. 2023. PMID: 37686236 Free PMC article.
-
Biochemical and Molecular Pathways in Neurodegenerative Diseases: An Integrated View.Cells. 2023 Sep 20;12(18):2318. doi: 10.3390/cells12182318. Cells. 2023. PMID: 37759540 Free PMC article. Review.
-
Single-molecule characterization of salivary protein aggregates from Parkinson's disease patients: a pilot study.Brain Commun. 2024 May 21;6(3):fcae178. doi: 10.1093/braincomms/fcae178. eCollection 2024. Brain Commun. 2024. PMID: 38863577 Free PMC article.
-
Alpha-Synuclein in Peripheral Tissues as a Possible Marker for Neurological Diseases and Other Medical Conditions.Biomolecules. 2023 Aug 18;13(8):1263. doi: 10.3390/biom13081263. Biomolecules. 2023. PMID: 37627328 Free PMC article. Review.
References
-
- Stewart T., Sui Y.T., Gonzalez-Cuyar L.F., Wong D.T., Akin D.M., Tumas V., Aasly J., Ashmore E., Aro P., Ginghina C., et al. Cheek cell-derived α-synuclein and DJ-1 do not differentiate Parkinson’s disease from control. Neurobiol. Aging. 2014;35:418–420. doi: 10.1016/j.neurobiolaging.2013.08.008. - DOI - PMC - PubMed
-
- Vivacqua G., Suppa A., Mancinelli R., Belvisi D., Fabbrini A., Costanzo M., Formica A., Onori P., Fabbrini G., Berardelli A. Salivary alpha-synuclein in the diagnosis of Parkinson’s disease and Progressive Supranuclear Palsy. Parkinsonism Relat. Disord. 2019;63:143–148. doi: 10.1016/j.parkreldis.2019.02.014. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

