Design, Synthesis, and Molecular Docking Study of New Tyrosyl-DNA Phosphodiesterase 1 (TDP1) Inhibitors Combining Resin Acids and Adamantane Moieties
- PMID: 34062881
- PMCID: PMC8147275
- DOI: 10.3390/ph14050422
Design, Synthesis, and Molecular Docking Study of New Tyrosyl-DNA Phosphodiesterase 1 (TDP1) Inhibitors Combining Resin Acids and Adamantane Moieties
Abstract
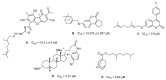
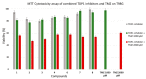
In this paper, a series of novel abietyl and dehydroabietyl ureas, thioureas, amides, and thioamides bearing adamantane moieties were designed, synthesized, and evaluated for their inhibitory activities against tyrosil-DNA-phosphodiesterase 1 (TDP1). The synthesized compounds were able to inhibit TDP1 at micromolar concentrations (0.19-2.3 µM) and demonstrated low cytotoxicity in the T98G glioma cell line. The effect of the terpene fragment, the linker structure, and the adamantane residue on the biological properties of the new compounds was investigated. Based on molecular docking results, we suppose that adamantane derivatives of resin acids bind to the TDP1 covalent intermediate, forming a hydrogen bond with Ser463 and hydrophobic contacts with the Phe259 and Trp590 residues and the oligonucleotide fragment of the substrate.
Keywords: TDP1; adamantane; resin acid; tyrosil-DNA-phosphodiesterase 1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Baglini E., Salerno S., Barresi E., Robello M., Da Settimo F., Taliani S., Marini A.M. Multiple Topoisomerase I (TopoI), Topoisomerase II (TopoII) and Tyrosyl-DNA Phosphodiesterase (TDP) inhibitors in the development of anticancer drugs. Eur. J. Pharm. Sci. 2021;156:105594. doi: 10.1016/j.ejps.2020.105594. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

