β-Boswellic Acid Inhibits RANKL-Induced Osteoclast Differentiation and Function by Attenuating NF-κB and Btk-PLCγ2 Signaling Pathways
- PMID: 34062884
- PMCID: PMC8125251
- DOI: 10.3390/molecules26092665
β-Boswellic Acid Inhibits RANKL-Induced Osteoclast Differentiation and Function by Attenuating NF-κB and Btk-PLCγ2 Signaling Pathways
Abstract
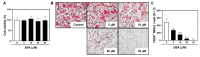
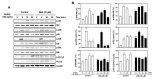
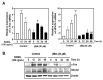
Osteoporosis is a systemic metabolic bone disorder that is caused by an imbalance in the functions of osteoclasts and osteoblasts and is characterized by excessive bone resorption by osteoclasts. Targeting osteoclast differentiation and bone resorption is considered a good fundamental solution for overcoming bone diseases. β-boswellic acid (βBA) is a natural compound found in Boswellia serrata, which is an active ingredient with anti-inflammatory, anti-rheumatic, and anti-cancer effects. Here, we explored the anti-resorptive effect of βBA on osteoclastogenesis. βBA significantly inhibited the formation of tartrate-resistant acid phosphatase-positive osteoclasts induced by receptor activator of nuclear factor-B ligand (RANKL) and suppressed bone resorption without any cytotoxicity. Interestingly, βBA significantly inhibited the phosphorylation of IκB, Btk, and PLCγ2 and the degradation of IκB. Additionally, βBA strongly inhibited the mRNA and protein expression of c-Fos and NFATc1 induced by RANKL and subsequently attenuated the expression of osteoclast marker genes, such as OC-STAMP, DC-STAMP, β3-integrin, MMP9, ATP6v0d2, and CtsK. These results suggest that βBA is a potential therapeutic candidate for the treatment of excessive osteoclast-induced bone diseases such as osteoporosis.
Keywords: bone resorption; osteoclast; osteoporosis; β-boswellic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
WHI-131 Promotes Osteoblast Differentiation and Prevents Osteoclast Formation and Resorption in Mice.J Bone Miner Res. 2016 Feb;31(2):403-15. doi: 10.1002/jbmr.2612. Epub 2015 Aug 29. J Bone Miner Res. 2016. PMID: 26255791
-
Stauntonia hexaphylla (Lardizabalaceae) leaf methanol extract inhibits osteoclastogenesis and bone resorption activity via proteasome-mediated degradation of c-Fos protein and suppression of NFATc1 expression.BMC Complement Altern Med. 2015 Aug 14;15:280. doi: 10.1186/s12906-015-0801-6. BMC Complement Altern Med. 2015. PMID: 26271279 Free PMC article.
-
Bajijiasu Abrogates Osteoclast Differentiation via the Suppression of RANKL Signaling Pathways through NF-κB and NFAT.Int J Mol Sci. 2017 Jan 19;18(1):203. doi: 10.3390/ijms18010203. Int J Mol Sci. 2017. PMID: 28106828 Free PMC article.
-
The Role of TAK1 in RANKL-Induced Osteoclastogenesis.Calcif Tissue Int. 2022 Jul;111(1):1-12. doi: 10.1007/s00223-022-00967-z. Epub 2022 Mar 14. Calcif Tissue Int. 2022. PMID: 35286417 Review.
-
Mechanisms involved in normal and pathological osteoclastogenesis.Cell Mol Life Sci. 2018 Jul;75(14):2519-2528. doi: 10.1007/s00018-018-2817-9. Epub 2018 Apr 18. Cell Mol Life Sci. 2018. PMID: 29670999 Free PMC article. Review.
Cited by
-
Geniposide Ameliorated Dexamethasone-Induced Cholesterol Accumulation in Osteoblasts by Mediating the GLP-1R/ABCA1 Axis.Cells. 2021 Dec 6;10(12):3424. doi: 10.3390/cells10123424. Cells. 2021. PMID: 34943934 Free PMC article.
-
SARS-CoV-2 E protein: Pathogenesis and potential therapeutic development.Biomed Pharmacother. 2023 Mar;159:114242. doi: 10.1016/j.biopha.2023.114242. Epub 2023 Jan 11. Biomed Pharmacother. 2023. PMID: 36652729 Free PMC article. Review.
-
Anti‑osteoclastogenic effect of fermented mealworm extract by inhibiting RANKL‑induced NFATc1 action.Exp Ther Med. 2024 Feb 6;27(4):130. doi: 10.3892/etm.2024.12418. eCollection 2024 Apr. Exp Ther Med. 2024. PMID: 38414787 Free PMC article.
-
Bioactive Compounds in Osteoarthritis: Molecular Mechanisms and Therapeutic Roles.Int J Mol Sci. 2024 Oct 30;25(21):11656. doi: 10.3390/ijms252111656. Int J Mol Sci. 2024. PMID: 39519204 Free PMC article. Review.
-
Recent advances of NFATc1 in rheumatoid arthritis-related bone destruction: mechanisms and potential therapeutic targets.Mol Med. 2024 Feb 3;30(1):20. doi: 10.1186/s10020-024-00788-w. Mol Med. 2024. PMID: 38310228 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

