Use of Procalcitonin during the First Wave of COVID-19 in the Acute NHS Hospitals: A Retrospective Observational Study
- PMID: 34062898
- PMCID: PMC8147337
- DOI: 10.3390/antibiotics10050516
Use of Procalcitonin during the First Wave of COVID-19 in the Acute NHS Hospitals: A Retrospective Observational Study
Abstract
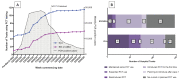
A minority of patients presenting to hospital with COVID-19 have bacterial co-infection. Procalcitonin testing may help identify patients for whom antibiotics should be prescribed or withheld. This study describes the use of procalcitonin in English and Welsh hospitals during the first wave of the COVID-19 pandemic. A web-based survey of antimicrobial leads gathered data about the use of procalcitonin testing. Responses were received from 148/151 (98%) eligible hospitals. During the first wave of the COVID-19 pandemic, there was widespread introduction and expansion of PCT use in NHS hospitals. The number of hospitals using PCT in emergency/acute admissions rose from 17 (11%) to 74/146 (50.7%) and use in Intensive Care Units (ICU) increased from 70 (47.6%) to 124/147 (84.4%). This increase happened predominantly in March and April 2020, preceding NICE guidance. Approximately half of hospitals used PCT as a single test to guide decisions to discontinue antibiotics and half used repeated measurements. There was marked variation in the thresholds used for empiric antibiotic cessation and guidance about interpretation of values. Procalcitonin testing has been widely adopted in the NHS during the COVID-19 pandemic in an unevidenced, heterogeneous way and in conflict with relevant NICE guidance. Further research is needed urgently that assesses the impact of this change on antibiotic prescribing and patient safety.
Keywords: COVID-19; antibiotic; procalcitonin; stewardship.
Conflict of interest statement
J.A.T.S. has current research funding relating to diagnostic testing from NIHR, MRC, EPSRC, Wellcome Trust, and Leeds Cares. Within the last 5 years, J.S. has been involved in research funded by Pfizer, Astellas, and Merck Sharp and Dohme. N.P. has received honoraria from Thermofisher. P.D. is ADAPT sepsis Chief Investigator. M.J.L., J.E., P.H., S.T. are investigators on PRONTO. M.A. has received lecturing fees from Pfizer, and lecturing fees from Shionogi, H.D.R. U.K. P.P. E.C. E.T.J. investigators on PRONTO, BATCH, PRECISE. T.H. I.M., investigators on ADAPT (RISC-sepsis) B.S. supported by the NIHR In Vitro diagnostics Co-operative. T.S., D.P., S.B., M.O., H.P., D.S., R.W., L.B.H., S.H. none to declare.
Figures
References
-
- World Health Organisation Weekly Operational Update on COVID-19-8 February 2021. [(accessed on 23 March 2021)]; Available online: https://www.who.int/publications/m/item/weekly-operational-update-on-cov....
-
- Gov.UK Coronovirus (COVID-19) in the UK. [(accessed on 25 February 2021)]; Available online: https://coronavirus.data.gov.uk/details/healthcare.
-
- Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., Satta G., Cooke G., Holmes A. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. - DOI - PMC - PubMed
-
- Martén-Loeches I.M.D.P., Sanchez-Corral A.M.D., Diaz E.M.D.P., Granada R.M.M.D., Zaragoza R.M.D., Villavicencio C.M.D., Albaya A.M.D., Cerdá E.M.D., Catalán R.M.M.D., Luque P.M.D., et al. Community-Acquired Respiratory Coinfection in Critically Ill Patients With Pandemic 2009 Influenza A(H1N1) Virus. Chest. 2011;139:555–562. doi: 10.1378/chest.10-1396. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

