High Carotenoid Mutants of Chlorella vulgaris Show Enhanced Biomass Yield under High Irradiance
- PMID: 34062906
- PMCID: PMC8147269
- DOI: 10.3390/plants10050911
High Carotenoid Mutants of Chlorella vulgaris Show Enhanced Biomass Yield under High Irradiance
Abstract
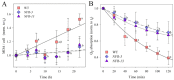
Microalgae represent a carbon-neutral source of bulk biomass, for extraction of high-value compounds and production of renewable fuels. Due to their high metabolic activity and reproduction rates, species of the genus Chlorella are highly productive when cultivated in photobioreactors. However, wild-type strains show biological limitations making algal bioproducts expensive compared to those extracted from other feedstocks. Such constraints include inhomogeneous light distribution due to high optical density of the culture, and photoinhibition of the surface-exposed cells. Thus, the domestication of algal strains for industry makes it increasingly important to select traits aimed at enhancing light-use efficiency while withstanding excess light stress. Carotenoids have a crucial role in protecting against photooxidative damage and, thus, represent a promising target for algal domestication. We applied chemical mutagenesis to Chlorella vulgaris and selected for enhanced tolerance to the carotenoid biosynthesis inhibitor norflurazon. The NFR (norflurazon-resistant) strains showed an increased carotenoid pool size and enhanced tolerance towards photooxidative stress. Growth under excess light revealed an improved carbon assimilation rate of NFR strains with respect to WT. We conclude that domestication of Chlorella vulgaris, by optimizing both carotenoid/chlorophyll ratio and resistance to photooxidative stress, boosted light-to-biomass conversion efficiency under high light conditions typical of photobioreactors. Comparison with strains previously reported for enhanced tolerance to singlet oxygen, reveals that ROS resistance in Chlorella is promoted by at least two independent mechanisms, only one of which is carotenoid-dependent.
Keywords: biomass; carotenoids; chloroplast; microalgae; norflurazon; photooxidative stress; photoprotection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Combined resistance to oxidative stress and reduced antenna size enhance light-to-biomass conversion efficiency in Chlorella vulgaris cultures.Biotechnol Biofuels. 2019 Sep 16;12:221. doi: 10.1186/s13068-019-1566-9. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 31534480 Free PMC article.
-
Chlorella vulgaris mutants with altered cell walls show increased permeability and enhanced extractability of intracellular molecules.Biotechnol Biofuels Bioprod. 2025 Jun 5;18(1):59. doi: 10.1186/s13068-025-02663-0. Biotechnol Biofuels Bioprod. 2025. PMID: 40474295 Free PMC article.
-
Domestication of the green alga Chlorella sorokiniana: reduction of antenna size improves light-use efficiency in a photobioreactor.Biotechnol Biofuels. 2014 Oct 21;7(1):157. doi: 10.1186/s13068-014-0157-z. eCollection 2014. Biotechnol Biofuels. 2014. PMID: 25352913 Free PMC article.
-
Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects.Crit Rev Food Sci Nutr. 2019;59(12):1880-1902. doi: 10.1080/10408398.2018.1432561. Epub 2018 Feb 16. Crit Rev Food Sci Nutr. 2019. PMID: 29370540 Review.
-
Production of carotenoids by microalgae: achievements and challenges.Photosynth Res. 2015 Sep;125(3):423-36. doi: 10.1007/s11120-015-0149-2. Epub 2015 Apr 29. Photosynth Res. 2015. PMID: 25921207 Review.
Cited by
-
Isolation and Selection of Protein-Rich Mutants of Chlorella vulgaris by Fluorescence-Activated Cell Sorting with Enhanced Biostimulant Activity to Germinate Garden Cress Seeds.Plants (Basel). 2024 Sep 1;13(17):2441. doi: 10.3390/plants13172441. Plants (Basel). 2024. PMID: 39273926 Free PMC article.
-
SL-6 Mimic Is a Biostimulant for Chlorella sorokiniana and Enhances the Plant Biostimulant Effect of Microalgal Extract.Plants (Basel). 2025 Mar 24;14(7):1010. doi: 10.3390/plants14071010. Plants (Basel). 2025. PMID: 40219078 Free PMC article.
-
Advances in light system engineering across the phototrophic spectrum.Front Plant Sci. 2024 Feb 12;15:1332456. doi: 10.3389/fpls.2024.1332456. eCollection 2024. Front Plant Sci. 2024. PMID: 38410727 Free PMC article. Review.
-
Proteomic characterization of a lutein-hyperaccumulating Chlamydomonas reinhardtii mutant reveals photoprotection-related factors as targets for increasing cellular carotenoid content.Biotechnol Biofuels Bioprod. 2023 Nov 4;16(1):166. doi: 10.1186/s13068-023-02421-0. Biotechnol Biofuels Bioprod. 2023. PMID: 37925447 Free PMC article.
-
Proteomic analysis of differential responses to norflurazon herbicide in the model green alga Chlamydomonas reinhardtii.Sci Rep. 2025 Aug 27;15(1):31601. doi: 10.1038/s41598-025-17119-6. Sci Rep. 2025. PMID: 40866566 Free PMC article.
References
-
- Weyer K.M., Bush D.R., Darzins A., Willson B.D. Theoretical maximum algal oil production. Bioenergy Res. 2010;3:204–213. doi: 10.1007/s12155-009-9046-x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

