The Kynurenic Acid Analog SZR72 Enhances Neuronal Activity after Asphyxia but Is Not Neuroprotective in a Translational Model of Neonatal Hypoxic Ischemic Encephalopathy
- PMID: 34062911
- PMCID: PMC8125407
- DOI: 10.3390/ijms22094822
The Kynurenic Acid Analog SZR72 Enhances Neuronal Activity after Asphyxia but Is Not Neuroprotective in a Translational Model of Neonatal Hypoxic Ischemic Encephalopathy
Abstract
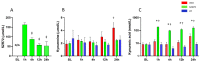
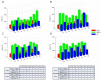
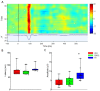
Hypoxic-ischemic encephalopathy (HIE) remains to be a major cause of long-term neurodevelopmental deficits in term neonates. Hypothermia offers partial neuroprotection warranting research for additional therapies. Kynurenic acid (KYNA), an endogenous product of tryptophan metabolism, was previously shown to be beneficial in rat HIE models. We sought to determine if the KYNA analog SZR72 would afford neuroprotection in piglets. After severe asphyxia (pHa = 6.83 ± 0.02, ΔBE = -17.6 ± 1.2 mmol/L, mean ± SEM), anesthetized piglets were assigned to vehicle-treated (VEH), SZR72-treated (SZR72), or hypothermia-treated (HT) groups (n = 6, 6, 6; Tcore = 38.5, 38.5, 33.5 °C, respectively). Compared to VEH, serum KYNA levels were elevated, recovery of EEG was faster, and EEG power spectral density values were higher at 24 h in the SZR72 group. However, instantaneous entropy indicating EEG signal complexity, depression of the visual evoked potential (VEP), and the significant neuronal damage observed in the neocortex, the putamen, and the CA1 hippocampal field were similar in these groups. In the caudate nucleus and the CA3 hippocampal field, neuronal damage was even more severe in the SZR72 group. The HT group showed the best preservation of EEG complexity, VEP, and neuronal integrity in all examined brain regions. In summary, SZR72 appears to enhance neuronal activity after asphyxia but does not ameliorate early neuronal damage in this HIE model.
Keywords: birth asphyxia; kynurenine; neonatal encephalopathy; newborn pig; therapeutic hypothermia.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures







Similar articles
-
Differential Effects of Hypothermia and SZR72 on Cerebral Kynurenine and Kynurenic Acid in a Piglet Model of Hypoxic-Ischemic Encephalopathy.Int J Mol Sci. 2023 Sep 25;24(19):14522. doi: 10.3390/ijms241914522. Int J Mol Sci. 2023. PMID: 37833970 Free PMC article.
-
Inhaled H2 or CO2 Do Not Augment the Neuroprotective Effect of Therapeutic Hypothermia in a Severe Neonatal Hypoxic-Ischemic Encephalopathy Piglet Model.Int J Mol Sci. 2020 Sep 16;21(18):6801. doi: 10.3390/ijms21186801. Int J Mol Sci. 2020. PMID: 32948011 Free PMC article.
-
Molecular hydrogen affords neuroprotection in a translational piglet model of hypoxic-ischemic encephalopathy.J Physiol Pharmacol. 2016 Oct;67(5):677-689. J Physiol Pharmacol. 2016. PMID: 28011948
-
Hydrogen-induced Neuroprotection in Neonatal Hypoxic-ischemic Encephalopathy.Curr Pharm Des. 2021;27(5):687-694. doi: 10.2174/1381612826666201113095720. Curr Pharm Des. 2021. PMID: 33185158 Review.
-
Hypoxic-ischemic encephalopathy in the newborn.Arch Neurol. 1983 May;40(5):261-6. doi: 10.1001/archneur.1983.04050050029002. Arch Neurol. 1983. PMID: 6405725 Review.
Cited by
-
Decoupling Behavioral Domains via Kynurenic Acid Analog Optimization: Implications for Schizophrenia and Parkinson's Disease Therapeutics.Cells. 2025 Jun 25;14(13):973. doi: 10.3390/cells14130973. Cells. 2025. PMID: 40643494 Free PMC article.
-
The effects of CO2 levels and body temperature on brain interstitial pH alterations during the induction of hypoxic-ischemic encephalopathy in newborn pigs.Heliyon. 2024 Mar 25;10(7):e28607. doi: 10.1016/j.heliyon.2024.e28607. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38571587 Free PMC article.
-
Differential Effects of Hypothermia and SZR72 on Cerebral Kynurenine and Kynurenic Acid in a Piglet Model of Hypoxic-Ischemic Encephalopathy.Int J Mol Sci. 2023 Sep 25;24(19):14522. doi: 10.3390/ijms241914522. Int J Mol Sci. 2023. PMID: 37833970 Free PMC article.
References
-
- Lee A.C.C., Kozuki N., Blencowe H., Vos T., Bahalim A., Darmstadt G.L., Niermeyer S., Ellis M., Robertson N.J., Cousens S., et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 2013;74:50–72. doi: 10.1038/pr.2013.206. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

