Frontline Screening for SARS-CoV-2 Infection at Emergency Department Admission by Third Generation Rapid Antigen Test: Can We Spare RT-qPCR?
- PMID: 34062916
- PMCID: PMC8147338
- DOI: 10.3390/v13050818
Frontline Screening for SARS-CoV-2 Infection at Emergency Department Admission by Third Generation Rapid Antigen Test: Can We Spare RT-qPCR?
Abstract
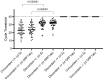
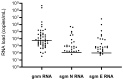
To complement RT-qPCR testing for diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, many countries have introduced the use of rapid antigen tests. As they generally display lower real-life performances than expected, their correct positioning as frontline screening is still controversial. Despite the lack of data from daily clinical use, third generation microfluidic assays (such as the LumiraDx SARS-CoV-2 Ag test) have recently been suggested to have similar performances to RT-qPCR and have been proposed as alternative diagnostic tools. By analyzing 960 nasopharyngeal swabs from 960 subjects at the emergency department admissions of a tertiary COVID-19 hospital, LumiraDx assay demonstrated a specificity of 97% (95% CI: 96-98), and a sensitivity of 85% (95% CI: 82-89) in comparison with RT-qPCR, which increases to 91% (95% CI: 86-95) for samples with a cycle threshold ≤ 29. Fifty false-negative LumiraDx-results were confirmed by direct quantification of genomic SARS-CoV-2 RNA through droplet-digital PCR (median (IQR) load = 5880 (1657-41,440) copies/mL). Subgenomic N and E RNAs were detected in 52% (n = 26) and 56% (n = 28) of them, respectively, supporting the presence of active viral replication. Overall, the LumiraDx test complies with the minimum performance requirements of the WHO. Yet, the risk of a misrecognition of patients with active COVID-19 persists, and the need for confirmatory RT-qPCR should not be amended.
Keywords: SARS-CoV-2; antigenic test; infectivity; rapid diagnostic test; subgenomic RNA; viral load.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bundesministerium für Soziales Gesundheit Pflege und Konsumentenschutz (BMSGMPK) Österreichische Teststrategie SARS-CoV-2. Bundesministerium für Soziales, Gesundheit, Pflege und Konsumentenschutz; Wien, Austria: 2020. [(accessed on 2 February 2020)]. Available online: https://www.sozialministerium.at/Informationen-zum-Coronavirus/Neuartige....
-
- Haute Autorité de Santé (HAS) Revue Rapide sur les Tests de Détection Antigénique du Virus SARS-CoV-2: HAS. [(accessed on 2 February 2021)];2020 Available online: https://www.has-sante.fr/jcms/p_3213483/fr/revue-rapide-sur-les-tests-de....
-
- Ministero della Salute (MS) Aggiornamento della Definizione di Caso COVID-19 e Strategie di Testing. [(accessed on 2 February 2021)];2021 Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&co....
-
- Rijskinstituut voor Volksgmezondheid en Milieu (RIVM) Advies Antigeen(snel)testen: Government of the Netherlands. [(accessed on 2 February 2021)];2020 Available online: https://www.rijksoverheid.nl/onderwerpen/coronavirus-covid-19/documenten....
-
- Robert Koch Institut (RKI) Nationale Teststrategie SARS-CoV-2. RKI; Berlin, Germany: 2020. [(accessed on 2 February 2021)]. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Teststrateg....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

