Fibrotic Events in the Progression of Cholestatic Liver Disease
- PMID: 34062960
- PMCID: PMC8147992
- DOI: 10.3390/cells10051107
Fibrotic Events in the Progression of Cholestatic Liver Disease
Abstract
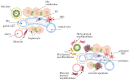
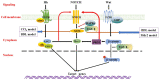
Cholestatic liver diseases including primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) are associated with active hepatic fibrogenesis, which can ultimately lead to the development of cirrhosis. However, the exact relationship between the development of liver fibrosis and the progression of cholestatic liver disease remains elusive. Periductular fibroblasts located around the bile ducts seem biologically different from hepatic stellate cells (HSCs). The fibrotic events in these clinical conditions appear to be related to complex crosstalk between immune/inflammatory mechanisms, cytokine signalling, and perturbed homeostasis between cholangiocytes and mesenchymal cells. Several animal models including bile duct ligation (BDL) and the Mdr2-knockout mice have improved our understanding of mechanisms underlying chronic cholestasis. In the present review, we aim to elucidate the mechanisms of fibrosis in order to help to identify potential diagnostic and therapeutic targets.
Keywords: cholangiocytes; cholestasis; fibrosis; hepatic stellate cells (HSCs); periductular fibroblasts.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Fuchs C.D., Halilbasic E., Trauner M. Pathophysiologic basis for alternative therapies for cholestasis. Liver Biol. Pathobiol. 2020:364–377. doi: 10.1002/9781119436812.ch30. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

