The Role of Sulphate and Phosphate Ions in the Recovery of Benzoic Acid Self-Enhanced Ozonation in Water Containing Bromides
- PMID: 34062968
- PMCID: PMC8125472
- DOI: 10.3390/molecules26092701
The Role of Sulphate and Phosphate Ions in the Recovery of Benzoic Acid Self-Enhanced Ozonation in Water Containing Bromides
Abstract
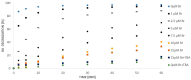
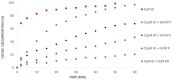
The ozonation of aromatic compounds in low-pH water is ineffective. In an acidic environment, the decomposition of ozone into hydroxyl radicals is limited and insufficient for the degradation of organic pollutants. Radical processes are also strongly inhibited by halogen ions present in the reaction medium, especially at low pH. It was shown that even under such unfavorable conditions, some compounds can initiate radical chain reactions leading to the formation of hydroxyl radicals, thus accelerating the ozonation process, which is referred to as so-called "self-enhanced ozonation". This paper presents the effect of bromides on "self-enhanced ozonation" of benzoic acid (BA) at pH 2.5. It is the first report to fully and quantitatively describe this process. The presence of only 15 µM bromides in water inhibits ozone decomposition and completely blocks BA degradation. However, the effectiveness of this process can be regained by ozonation in the presence of phosphates or sulphate. The addition of these inorganic salts to the bromide-containing solution helps to recover ozone decomposition and BA degradation efficiency. As part of this research, the fractions of hydroxyl, sulphate and phosphate radicals reacting with benzoic acid and bromides were calculated.
Keywords: advanced oxidation processes; bromides; ozonation; phosphates; radicals; sulphate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Nawrocki J., Kasprzyk-Hordern B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B Environ. 2010;99:27–42. doi: 10.1016/j.apcatb.2010.06.033. - DOI
-
- Nawrocki J., Fijołek L. Mechanisms and Efficiency of Catalytic Ozonation in Water Treatment. Ochr. Srodowiska. 2009;31:3–16.
LinkOut - more resources
Full Text Sources
Research Materials

