Functional Pyrazolo[1,5- a]pyrimidines: Current Approaches in Synthetic Transformations and Uses As an Antitumor Scaffold
- PMID: 34063043
- PMCID: PMC8125733
- DOI: 10.3390/molecules26092708
Functional Pyrazolo[1,5- a]pyrimidines: Current Approaches in Synthetic Transformations and Uses As an Antitumor Scaffold
Abstract
Pyrazolo[1,5-a]pyrimidine (PP) derivatives are an enormous family of N-heterocyclic compounds that possess a high impact in medicinal chemistry and have attracted a great deal of attention in material science recently due to their significant photophysical properties. Consequently, various researchers have developed different synthesis pathways for the preparation and post-functionalization of this functional scaffold. These transformations improve the structural diversity and allow a synergic effect between new synthetic routes and the possible applications of these compounds. This contribution focuses on an overview of the current advances (2015-2021) in the synthesis and functionalization of diverse pyrazolo[1,5-a]pyrimidines. Moreover, the discussion highlights their anticancer potential and enzymatic inhibitory activity, which hopefully could lead to new rational and efficient designs of drugs bearing the pyrazolo[1,5-a]pyrimidine core.
Keywords: N-heterocyclic compounds; antitumor scaffold; enzymatic inhibitory; functionalization; organic synthesis; pyrazolo[1,5-a]pyrimidine.
Conflict of interest statement
The authors declare no conflict of interest because of this literature research was conducted in the absence of any commercial or financial relationships.
Figures





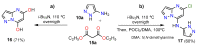
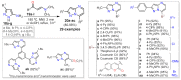




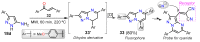

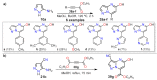
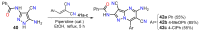

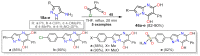








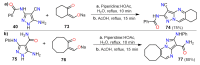
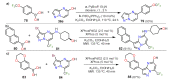




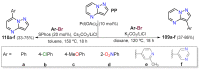

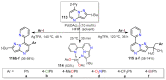
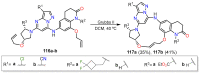

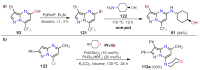




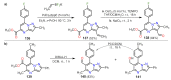








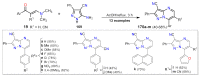


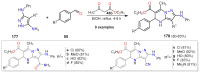





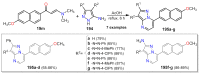
References
-
- Castillo J.C., Portilla J. Recent advances in the synthesis of new pyrazole derivatives. Targets Heterocycl. Syst. 2018;22:194–223. doi: 10.17374/targets.2019.22.194. - DOI
-
- Salem M.A., Helal M.H., Gouda M.A., Abd EL-Gawad H.H., Shehab M.A.M., El-Khalafawy A. Recent synthetic methodologies for pyrazolo[1,5-a]pyrimidine. Synth. Commun. 2019;49:1750–1776. doi: 10.1080/00397911.2019.1604967. - DOI
-
- Al-Azmi A. Pyrazolo[1,5-a]pyrimidines: A Close Look into their Synthesis and Applications. Curr. Org. Chem. 2019;23:721–743. doi: 10.2174/1385272823666190410145238. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

