Genetic and Epigenomic Modifiers of Diabetic Neuropathy
- PMID: 34063061
- PMCID: PMC8124699
- DOI: 10.3390/ijms22094887
Genetic and Epigenomic Modifiers of Diabetic Neuropathy
Abstract
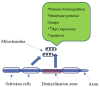
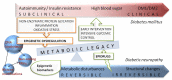
Diabetic neuropathy (DN), the most common chronic and progressive complication of diabetes mellitus (DM), strongly affects patients' quality of life. DN could be present as peripheral, autonomous or, clinically also relevant, uremic neuropathy. The etiopathogenesis of DN is multifactorial, and genetic components play a role both in its occurrence and clinical course. A number of gene polymorphisms in candidate genes have been assessed as susceptibility factors for DN, and most of them are linked to mechanisms such as reactive oxygen species production, neurovascular impairments and modified protein glycosylation, as well as immunomodulation and inflammation. Different epigenomic mechanisms such as DNA methylation, histone modifications and non-coding RNA action have been studied in DN, which also underline the importance of "metabolic memory" in DN appearance and progression. In this review, we summarize most of the relevant data in the field of genetics and epigenomics of DN, hoping they will become significant for diagnosis, therapy and prevention of DN.
Keywords: diabetic neuropathy; epigenetics; gene polymorphisms; genetic markers.
Conflict of interest statement
Authors declare no conflict of interest.
Figures

References
-
- Kallinikou D., Soldatou A., Tsentidis C., Louraki M., Kanaka-Gantenbein C., Kanavakis E., Karavanaki K. Diabetic Neuropathy in Children and Adolescents with Type 1 Diabetes Mellitus: Diagnosis, Pathogenesis, and Associated Genetic Markers. Diabetes. Metab. Res. Rev. 2019;35:e3178. doi: 10.1002/dmrr.3178. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

