Preventive Applications of Polyphenols in Dentistry-A Review
- PMID: 34063086
- PMCID: PMC8124254
- DOI: 10.3390/ijms22094892
Preventive Applications of Polyphenols in Dentistry-A Review
Abstract
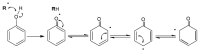
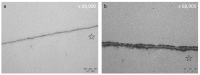
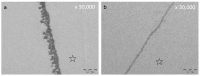
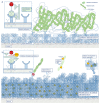
Polyphenols are natural substances that have been shown to provide various health benefits. Antioxidant, anti-inflammatory, and anti-carcinogenic effects have been described. At the same time, they inhibit the actions of bacteria, viruses, and fungi. Thus, studies have also examined their effects within the oral cavity. This review provides an overview on the different polyphenols, and their structure and interactions with the tooth surface and the pellicle. In particular, the effects of various tea polyphenols on bioadhesion and erosion have been reviewed. The current research confirms that polyphenols can reduce the growth of cariogenic bacteria. Furthermore, they can decrease the adherence of bacteria to the tooth surface and improve the erosion-protective properties of the acquired enamel pellicle. Tea polyphenols, especially, have the potential to contribute to an oral health-related diet. However, in vitro studies have mainly been conducted. In situ studies and clinical studies need to be extended and supplemented in order to significantly contribute to additive prevention measures in caries prophylaxis.
Keywords: medicinal plants; molecular mechanisms; polyphenols; preventive dentistry; salivary pellicle; tea drugs; transmission electron microscopy (TEM).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



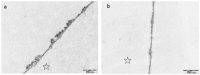
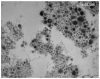
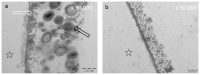
References
-
- Ditano-Vazquez P., Torres-Pena J.D., Galeano-Valle F., Perez-Caballero A.I., Demelo-Rodriguez P., Lopez-Miranda J., Katsiki N., Delgado-Lista J., Alvarez-Sala-Walther L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients. 2019;11:2833. doi: 10.3390/nu11112833. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

