The Influence of an Adrenergic Antagonist Guanethidine on the Distribution Pattern and Chemical Coding of Caudal Mesenteric Ganglion Perikarya and Their Axons Supplying the Porcine Bladder
- PMID: 34063103
- PMCID: PMC8124201
- DOI: 10.3390/ijms22094896
The Influence of an Adrenergic Antagonist Guanethidine on the Distribution Pattern and Chemical Coding of Caudal Mesenteric Ganglion Perikarya and Their Axons Supplying the Porcine Bladder
Abstract
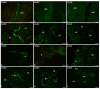
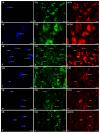
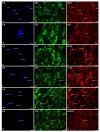
This study was aimed at disclosing the influence of intravesically instilled guanethidine (GUA) on the distribution, relative frequency and chemical coding of both the urinary bladder intramural sympathetic nerve fibers and their parent cell bodies in the caudal mesenteric ganglion (CaMG) in juvenile female pigs. GUA instillation led to a profound decrease in the number of perivascular nerve terminals. Furthermore, the chemical profile of the perivascular innervation within the treated bladder also distinctly changed, as most of axons became somatostatin-immunoreactive (SOM-IR), while in the control animals they were found to be neuropeptide Y (NPY)-positive. Intravesical treatment with GUA led not only to a significant decrease in the number of bladder-projecting tyrosine hydroxylase (TH) CaMG somata (94.3 ± 1.8% vs. 73.3 ± 1.4%; control vs. GUA-treated pigs), but simultaneously resulted in the rearrangement of their co-transmitters repertoire, causing a distinct decrease in the number of TH+/NPY+ (89.6 ± 0.7% vs. 27.8 ± 0.9%) cell bodies and an increase in the number of SOM-(3.6 ± 0.4% vs. 68.7 ± 1.9%), calbindin-(CB; 2.06 ± 0.2% vs. 9.1 ± 1.2%) or galanin-containing (GAL; 1.6 ± 0.3% vs. 28.2 ± 1.3%) somata. The present study provides evidence that GUA significantly modifies the sympathetic innervation of the porcine urinary bladder wall, and thus may be considered a potential tool for studying the plasticity of this subdivision of the bladder innervation.
Keywords: guanethidine; inferior mesenteric ganglion; neuropeptides; noradrenergic nerve fibers; pig; urinary bladder.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
The Influence of an Adrenergic Antagonist Guanethidine (GUA) on the Distribution Pattern and Chemical Coding of Dorsal Root Ganglia (DRG) Neurons Supplying the Porcine Urinary Bladder.Int J Mol Sci. 2021 Dec 13;22(24):13399. doi: 10.3390/ijms222413399. Int J Mol Sci. 2021. PMID: 34948196 Free PMC article.
-
The Influence of Tetrodotoxin (TTX) on the Distribution and Chemical Coding of Caudal Mesenteric Ganglion (CaMG) Neurons Supplying the Porcine Urinary Bladder.Mar Drugs. 2017 Mar 30;15(4):101. doi: 10.3390/md15040101. Mar Drugs. 2017. PMID: 28358321 Free PMC article.
-
The influence of resiniferatoxin on the chemical coding of caudal mesenteric ganglion neurons supplying the urinary bladder in the pig.J Physiol Pharmacol. 2016 Aug;67(4):625-632. J Physiol Pharmacol. 2016. PMID: 27779483
-
Reduction of the number of neurones in the caudal mesenteric ganglion innervating the ovary in sexually mature gilts following testosterone administration.J Neuroendocrinol. 2013 Sep;25(9):826-38. doi: 10.1111/jne.12057. J Neuroendocrinol. 2013. PMID: 23763306
-
Immunohistochemical characteristics and distribution of neurons in the paravertebral, prevertebral and pelvic ganglia supplying the urinary bladder in the male pig.J Mol Neurosci. 2014 Jan;52(1):56-70. doi: 10.1007/s12031-013-0139-9. J Mol Neurosci. 2014. PMID: 24122239
Cited by
-
Effect of regional crosstalk between sympathetic nerves and sensory nerves on temporomandibular joint osteoarthritic pain.Int J Oral Sci. 2025 Jan 7;17(1):3. doi: 10.1038/s41368-024-00336-6. Int J Oral Sci. 2025. PMID: 39762209 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

