BCG Cell Wall Skeleton As a Vaccine Adjuvant Protects Both Infant and Old-Aged Mice from Influenza Virus Infection
- PMID: 34063125
- PMCID: PMC8148143
- DOI: 10.3390/biomedicines9050516
BCG Cell Wall Skeleton As a Vaccine Adjuvant Protects Both Infant and Old-Aged Mice from Influenza Virus Infection
Abstract
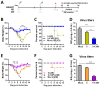
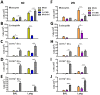
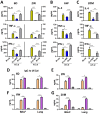
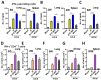
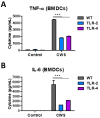
Bacillus Calmette-Guerin (BCG) and the cell wall skeleton (CWS) derived from BCG are known to enhance nonspecific immune activation and anti-cancer immunity; however, their roles as a vaccine adjuvant are largely unknown. Here, we report that BCG-CWS acts as a strong immune adjuvant by promoting the protective immune responses in mouse models with influenza vaccination. The different aged mice immunized with inactivated split vaccine with or without BCG-CWS were challenged with an influenza pandemic virus. When protective immune responses were compared, even a single immunization of adult mice with a BCG-CWS-adjuvanted vaccine showed significantly enhanced humoral immune responses with increased IgG1 and IgG2a isotype antibodies. Importantly, the protective effects by the BCG-CWS adjuvant for influenza vaccination upon humoral and cellular immunogenicity were comparable between infants (6 days and 2 weeks old) and aged (20 months old) mice. Moreover, BCG-CWS dramatically augmented vaccine-mediated protective responses, including decreased viral loads, lung damage, and airway resistance, as well as increased mouse survival, amelioration of weight loss, and proinflammatory cytokine expression in all experimental groups including infant, adults, and old aged mice. We further provided the evidence that the BCG-CWS adjuvant effects were mediated through Toll-like receptors (TLR) 2 and TLR4 signaling pathways. Together, these data suggest that BCG-CWS can be promising as a potential influenza vaccine adjuvant in both young and old aged population through TLR2/4-mediated immune-boosting activities.
Keywords: cell wall skeleton (CWS) adjuvant; immunological protective immunity; influenza virus; split vaccine.
Conflict of interest statement
The authors declare that there are no competing interests.
Figures



Similar articles
-
Enhancing activity of mycobacterial cell-derived adjuvants on immunogenicity of recombinant human hepatitis B virus vaccine.Vaccine. 1998 Dec;16(20):1982-9. doi: 10.1016/s0264-410x(98)00084-x. Vaccine. 1998. PMID: 9796054
-
Immune adjuvant therapy using Bacillus Calmette-Guérin cell wall skeleton (BCG-CWS) in advanced malignancies: A phase 1 study of safety and immunogenicity assessments.Medicine (Baltimore). 2019 Aug;98(33):e16771. doi: 10.1097/MD.0000000000016771. Medicine (Baltimore). 2019. PMID: 31415377 Free PMC article. Clinical Trial.
-
Cell wall skeleton of Mycobacterium bovis BCG enhances the vaccine potential of antigen 85B against tuberculosis by inducing Th1 and Th17 responses.PLoS One. 2019 Mar 8;14(3):e0213536. doi: 10.1371/journal.pone.0213536. eCollection 2019. PLoS One. 2019. PMID: 30849108 Free PMC article.
-
Bladder Cancer Medication Bacillus Calmette-Guérin-Cell Wall Skeleton Focusing on Alternatives and Developments to Limitations.J Cancer Prev. 2025 Mar 30;30(1):1-6. doi: 10.15430/JCP.25.002. J Cancer Prev. 2025. PMID: 40201027 Free PMC article. Review.
-
Development of immunoadjuvants for immunotherapy of cancer.Int Immunopharmacol. 2001 Jul;1(7):1249-59. doi: 10.1016/s1567-5769(01)00055-8. Int Immunopharmacol. 2001. PMID: 11460306 Review.
Cited by
-
Comparison of the effects of different potent adjuvants on enhancing the immunogenicity and cross-protection by influenza virus vaccination in young and aged mice.Antiviral Res. 2022 Jan;197:105229. doi: 10.1016/j.antiviral.2021.105229. Epub 2021 Dec 18. Antiviral Res. 2022. PMID: 34933043 Free PMC article.
-
Physical radiofrequency adjuvant enhances immune responses to influenza H5N1 vaccination.FASEB J. 2022 Mar;36(3):e22182. doi: 10.1096/fj.202101703R. FASEB J. 2022. PMID: 35113455 Free PMC article.
-
Immunostimulatory effects of marine algae extracts on in vitro antigen-presenting cell activation and in vivo immune cell recruitment.Food Sci Nutr. 2023 Aug 21;11(10):6560-6570. doi: 10.1002/fsn3.3605. eCollection 2023 Oct. Food Sci Nutr. 2023. PMID: 37823147 Free PMC article.
-
Exploiting bacterial-origin immunostimulants for improved vaccination and immunotherapy: current insights and future directions.Cell Biosci. 2024 Feb 17;14(1):24. doi: 10.1186/s13578-024-01207-7. Cell Biosci. 2024. PMID: 38368397 Free PMC article. Review.
-
The Adjuvant Activity of BCG Cell Wall Cytoskeleton on a Dengue Virus-2 Subunit Vaccine.Vaccines (Basel). 2023 Aug 9;11(8):1344. doi: 10.3390/vaccines11081344. Vaccines (Basel). 2023. PMID: 37631912 Free PMC article.
References
-
- Arts R.J., Moorlag S.J., Novakovic B., Li Y., Wang S.-Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A., et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe. 2018;23:89–100.e5. doi: 10.1016/j.chom.2017.12.010. - DOI - PubMed
-
- Covián C., Fernández-Fierro A., Retamal-Díaz A., Díaz F.E., Vasquez A.E., Lay M.K., Riedel C.A., González P.A., Bueno S.M., Kalergis A.M. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019;10:2806. doi: 10.3389/fimmu.2019.02806. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

