Adipose Tissue-Derived Stem Cell Yield Depends on Isolation Protocol and Cell Counting Method
- PMID: 34063138
- PMCID: PMC8148142
- DOI: 10.3390/cells10051113
Adipose Tissue-Derived Stem Cell Yield Depends on Isolation Protocol and Cell Counting Method
Abstract
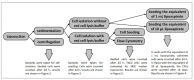
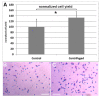
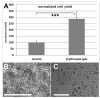
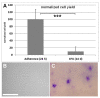
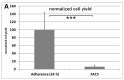
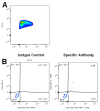
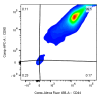
In plastic surgery, lipofilling is a frequent procedure. Unsatisfactory vascularization and impaired cell vitality can lead to unpredictable take rates in the fat graft. The proliferation and neovascularization inducing properties of adipose tissue-derived stem cells may contribute to solve this problem. Therefore, the enrichment of fat grafts with stem cells is studied intensively. However, it is difficult to compare these studies because many factors-often not precisely described-are influencing the results. Our study summarizes some factors which influence the cell yield like harvesting, isolation procedure and quantification. Stem cells were isolated after liposuction. Quantification was done using a cell chamber, colony counting, or flow cytometry with changes to one parameter, only, for each comparison. Quantification of cells isolated after liposuction at the same harvesting site from the same patient can vary greatly depending on the details of the isolation protocol and the method of quantification. Cell yield can be influenced strongly by many factors. Therefore, a comparison of different studies should be handled with care.
Keywords: adipocyte viability; adipose tissue; cell yield; fat grafting; lipoaspirate; plastic surgery; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

