Safety and Efficacy of Early High Parenteral Lipid Supplementation in Preterm Infants: A Systematic Review and Meta-Analysis
- PMID: 34063216
- PMCID: PMC8147506
- DOI: 10.3390/nu13051535
Safety and Efficacy of Early High Parenteral Lipid Supplementation in Preterm Infants: A Systematic Review and Meta-Analysis
Abstract
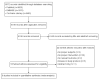
The objective of this systematic review and meta-analysis was to summarize the effects of early initiation and achievement of a high dose of parenteral lipids (≥1.5 g/kg/day reached within the first 24 h of birth) on growth and adverse outcomes in preterm infants. PubMed, EMBASE, and Cochrane databases were utilized to search for publications for this meta-analysis. Randomized controlled trials were eligible if data on growth or clinical outcome was available. The search returned nine studies. The mean proportion of postnatal weight loss (%) was lower (mean difference [MD]: -2.73; 95% confidence interval [CI]: -3.69, -1.78), and the mean head circumference near the term equivalent age (cm) was higher in the early high lipid treatment group (MD: 0.67; 95% CI: 0.25, 1.09). There was a favorable association of early high lipid administration with the incidence of extrauterine growth restriction (relative risk [RR]: 0.27; 95% CI: 0.15, 0.48). Generally, there were no differences in morbidities or adverse outcomes with early high lipid administration. Early initiation of parenteral lipids and high dose achieved within the first 24 h of life appear to be safe and endurable and offer benefits in terms of growth.
Keywords: adverse effect; growth; lipids; preterm infants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Low- versus High-Dose and Early versus Late Parenteral Amino-Acid Administration in Very-Low-Birth-Weight Infants: A Systematic Review and Meta-Analysis.Neonatology. 2018;113(3):187-205. doi: 10.1159/000481192. Epub 2017 Dec 22. Neonatology. 2018. PMID: 29268262
-
Intravenous lipids in preterm infants: impact on laboratory and clinical outcomes and long-term consequences.World Rev Nutr Diet. 2015;112:71-80. doi: 10.1159/000365459. Epub 2014 Nov 24. World Rev Nutr Diet. 2015. PMID: 25471804 Review.
-
Early parenteral lipids and growth velocity in extremely-low-birth-weight infants.Clin Nutr. 2014 Jun;33(3):502-8. doi: 10.1016/j.clnu.2013.07.007. Epub 2013 Jul 18. Clin Nutr. 2014. PMID: 23958274
-
Probiotics for prevention of necrotizing enterocolitis in preterm infants.Evid Based Child Health. 2014 Sep;9(3):584-671. doi: 10.1002/ebch.1976. Evid Based Child Health. 2014. PMID: 25236307 Review.
-
Does intravenous fish oil affect the growth of extremely low birth weight preterm infants on parenteral nutrition?Clin Nutr. 2019 Oct;38(5):2319-2324. doi: 10.1016/j.clnu.2018.10.009. Epub 2018 Oct 16. Clin Nutr. 2019. PMID: 30392754
Cited by
-
[Expert consensus on parenteral nutrition management in neonates (2025)].Zhongguo Dang Dai Er Ke Za Zhi. 2025 Mar 15;27(3):247-261. doi: 10.7499/j.issn.1008-8830.2411156. Zhongguo Dang Dai Er Ke Za Zhi. 2025. PMID: 40105069 Free PMC article. Chinese.
-
Dilemmas in the delivery of intravenous lipid emulsions and approach to hypertriglyceridemia in very preterm and low birth weight infants.J Perinatol. 2023 Sep;43(9):1189-1193. doi: 10.1038/s41372-023-01637-0. Epub 2023 Apr 8. J Perinatol. 2023. PMID: 37031340 Review.
-
ROP: 80 Years after Its Detection - Where Do We Stand and How Long Will We Continue to Laser?Neonatology. 2024;121(5):608-615. doi: 10.1159/000538907. Epub 2024 May 22. Neonatology. 2024. PMID: 38776885 Free PMC article. Review.
-
Nutritional interventions to prevent retinopathy of prematurity.Pediatr Res. 2024 Sep;96(4):905-911. doi: 10.1038/s41390-024-03208-1. Epub 2024 Apr 29. Pediatr Res. 2024. PMID: 38684884 Free PMC article. Review.
-
Nutritional Management for Preterm Infants with Common Comorbidities: A Narrative Review.Nutrients. 2025 Jun 9;17(12):1959. doi: 10.3390/nu17121959. Nutrients. 2025. PMID: 40573070 Free PMC article. Review.
References
-
- Simmer K., Rao S.C. Early introduction of lipids to parenterally-fed preterm infants. Cochrane Database Syst. Rev. 2005;18:CD005256. - PubMed
-
- Vlaardingerbroek H., Veldhorst M.A., Spronk S., van den Akker C.H., van Goudoever J.B. Parenteral lipid administration to very-low-birth-weight infants—early introduction of lipids and use of new lipid emulsions: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012;96:255–268. doi: 10.3945/ajcn.112.040717. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

